Overview of ADC-Platform
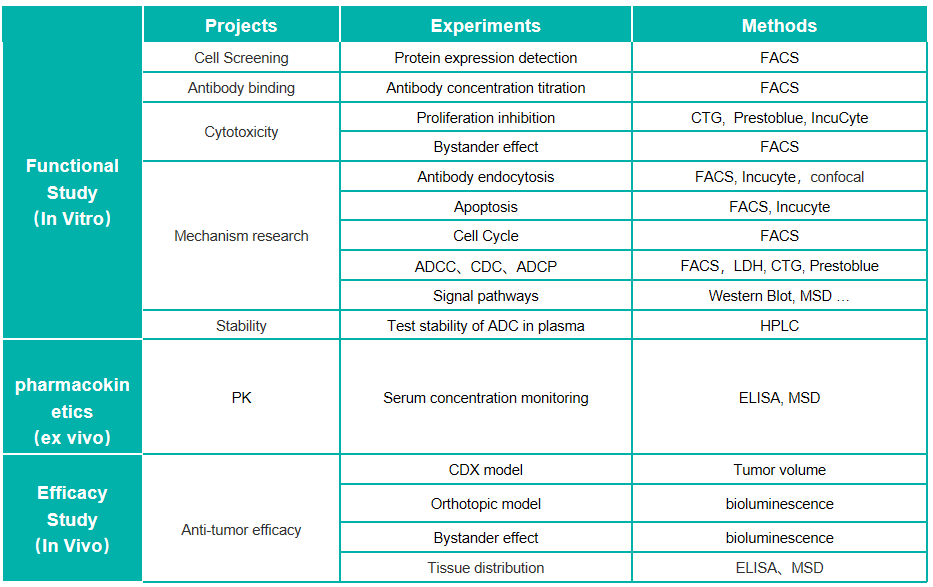
Antibody–drug conjugates (ADCs), one of novel therapeutical biologics, are composed of a monoclonal antibody that recognizes a specific tumor antigen, an extremely potent cytotoxic drug known as payload, and a linker connecting the payload to the antibody, aiming to take advantage of the specificity of monoclonal antibodies to deliver potent cytotoxic drugs selectively to antigen-expressing tumor cells. Recently, ADCs have been considered as an important means of future disease treatment and the global upsurge of that has been risen up. We have established a scientific ADC-related platform to support the evaluation of the efficacy of ADCs as well as the combination strategy of ADCs and other anti-cancer therapy for preclinical research of both McAb-ADC and BiAb-ADC.
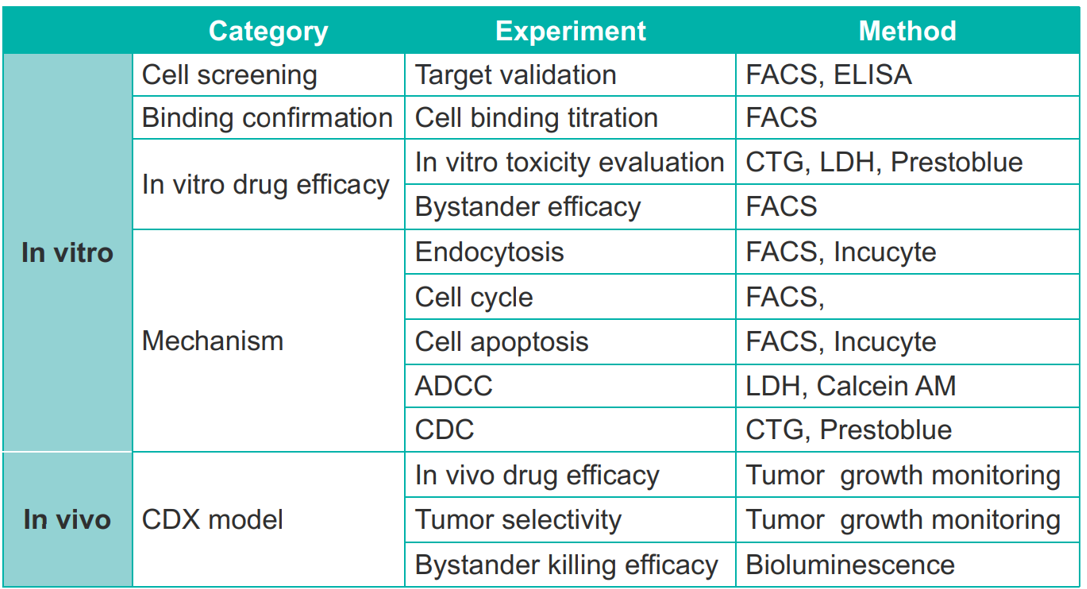
We have established a state-of-the-art pharmacology platform to study the mechanism of action and the functional effects of ADC for supporting the application of the investigational new drug. Our flow cytometry platform and cell base platform support a series of in-vitro studies in a quick, reliable, and reproducible way, including the evaluation of affinity and binding, the process of internalization, payload efficacy(apoptosis and cell cycle), as well as antibody fragment efficacy (ADCC, CDC, ADCP).In terms of in vivo ADCs assessment, our full-fledged team and abundant CDX resource also efficiently support the efficacy evaluation of ADCs or combination treatment. Also, we are proficient in constructing customized antigen-specific overexpression or knock-in cells used for the development of ADCs which theoretically target the specific antigens
Results
Target binding affinity validation assay
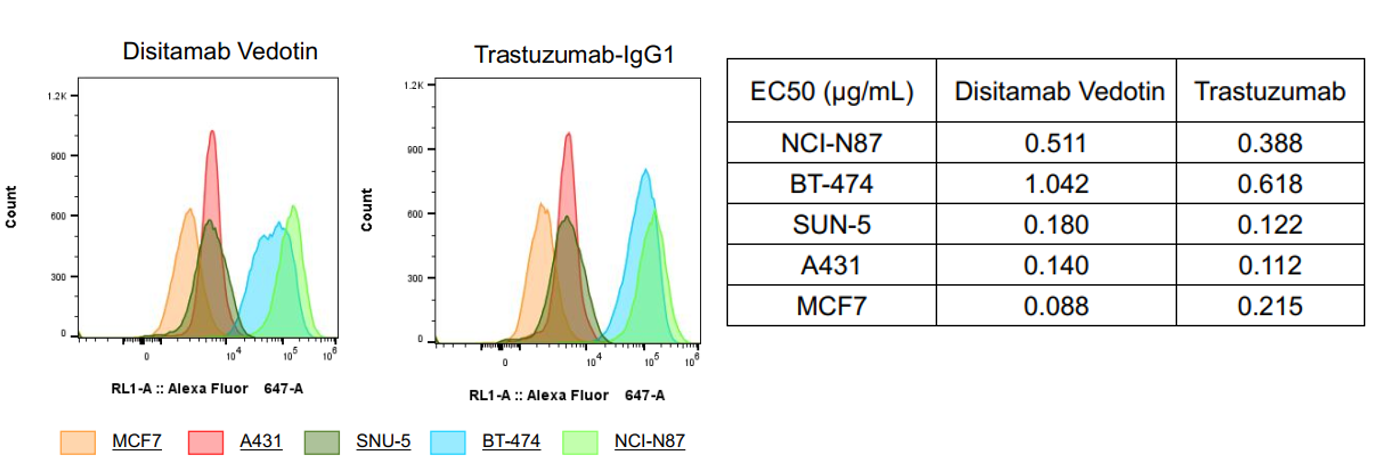
Fig1. Target binding affinity validation by flow cytometry. Disitamab Vedotin (RC-48) and trastuzumab have higher binding affinity to BT-474 and NCI-N87 cells compared to MCF7, A431, or SNU-5 cell lines. The EC50s are shown in the above table.
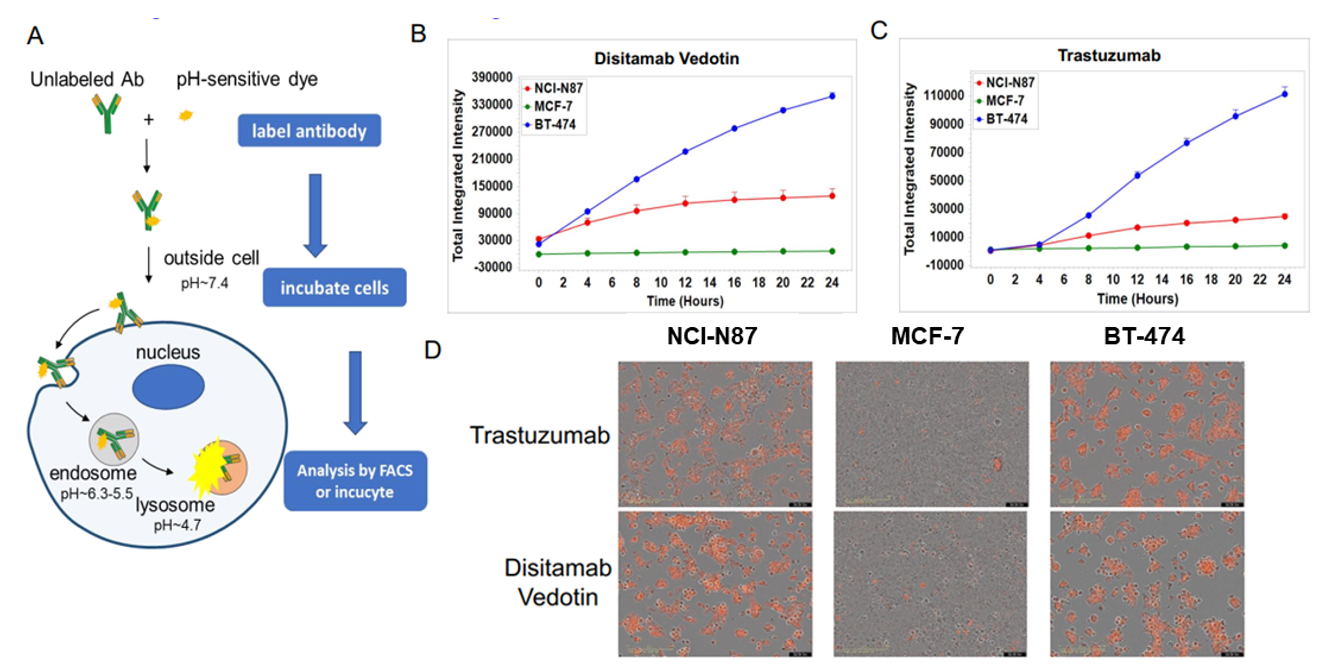
Fig2. In vitro internalization assay of Disitamab Vedotin and trastuzumab. A: The schematic diagram of antibody internalization assay. NCI-N87, MCF-7, and BT-474 cells were incubated with pH-sensitive-dye-labeled disitamab vedotin(RC-48) or trastuzumab for 24hr and observed continuously with Incucyte. B-C: The ordinate indicates the quantity of intracellular antibodies. D: The orange signals in the photos indicate the internalized antibodies. Both disitamab vedotin(RC-48) and trastuzumab were internalized into NCI-N87 and BT-474 cells in a time-dependent manner, whereas not in MCF-7 cells.
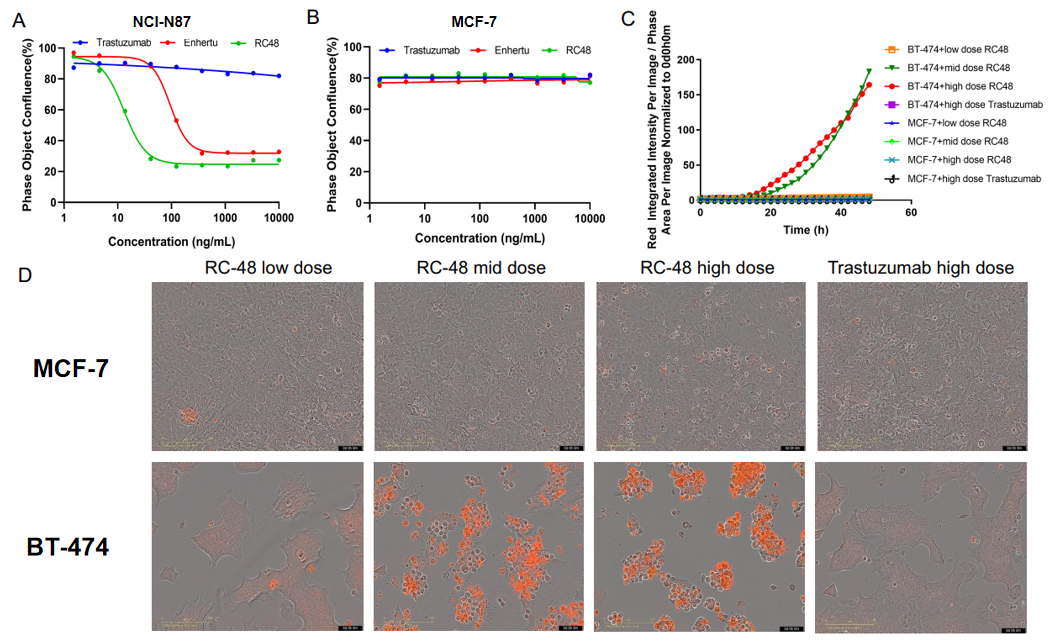
Fig3. In vitro cell proliferation and apoptosis assays to evaluate payload effects. A-B: NCI-N87 or MCF-7 cells were treated with varying concentrations of Trastuzumab, Enhertu, or Disitamab Vedotin (RC-48). 6 days later, the confluence of cells was calculated with Incucyte. Enhertu or RC-48 significantly inhibited NCI-N87 proliferation in a concentration-dependent manner, while trastuzumab was not. In addition, all of them could not inhibit MCF-7 proliferation, which presents a very low expression of HER2. C-D: BT-474 or MCF-7 cells were treated with different concentrations of trastuzumab or RC-48 for 72h. Apoptosis was detected with Sartorius IncuCyte Caspase-3/7 red Apoptosis Regent. Red signals indicate the activated caspase 3/7. RC-48(mid or high dose) significantly promoted BT-474 apoptosis, while trastuzumab did not. Similarly, apoptosis was not observed on treated MCF-7 cells.
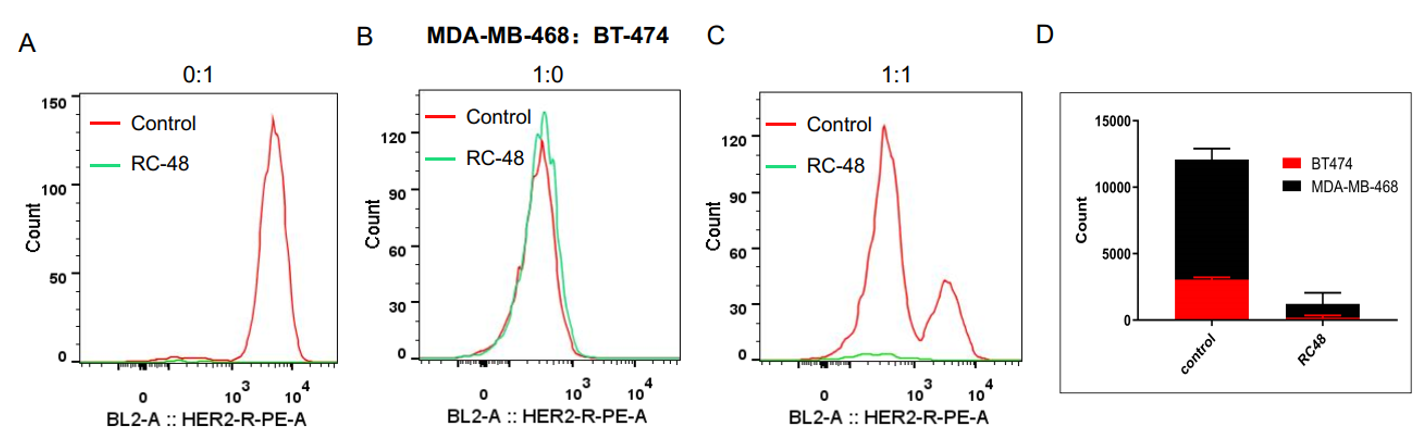
Fig 4. Bystander killing effect assay of Disitamab Vedotin (RC-48) in coculture conditions in vitro. MDA-MB-468 cells (HER2 negative) and BT-474 (HER2 positive) were cocultured overnight in different proportion (respectively 0:1, 1:0, 1:1). After being treated with Disitamab Vedotin (RC-48) or vehicle for 5 days, cell number and the ratio of HER2-positive: HER2-negative cells were determined by cell counter and flow cytometer, respectively. RC-48 showed great tumoricidal effects for BT-474 cells (A), while almost no cytotoxicity for MDA-MB-468 cells (B). Importantly, Disitamab Vedotin (RC-48) could also kill MDA-MB-468 cells when they were cocultured with HER2-positive cells (C-D) .
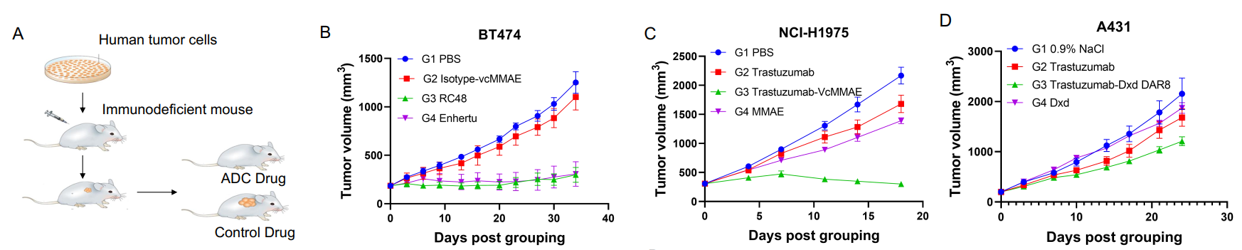
Fig 5. In vivo anti-tumor efficacy study of ADCs in several CDX models. A: The schematic diagram of in vivo assay. Human tumor cell lines were inoculated into immunodeficient mice at an exactly cell concentration. Mice were randomly regrouped when tumor volume reached 200 mm3 and treated with different ADCs (3mg/kg) or corresponding quantity of antibodies and payloads. B-D: All of the tested ADCs showed significant inhibition against tumor growth, especially in BT-474 and NCI-H1975 models (B-C). And compromised inhibition on the A431 model was observed (D), which is consistent with the low expression of HER2 on A431 cells (As shown in Figure) .
Antibody conjugate—ADC
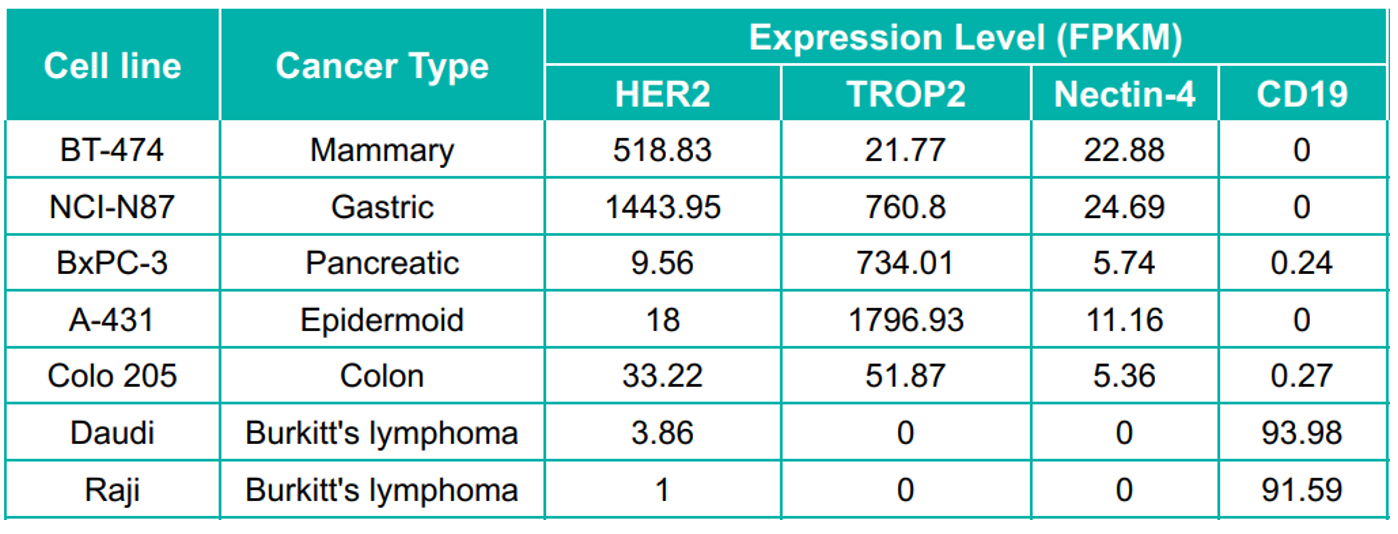
Recommended cell lines and related animals models






 +86-10-56967680
+86-10-56967680 info@bbctg.com.cn
info@bbctg.com.cn