Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that leads to encephalitis and demyelination. MS is considered an autoimmune disease caused by autoreactive T cells with symptoms including muscle rigidity and paralysis, visual disturbance and blindness, sensory loss and ataxia, and is characterized by recurrent relapses. At present, there are many different animal models of MS, of which the experimental autoimmune encephalomyelitis (EAE) model is widely used in the study of multiple sclerosis due to its pathological characteristics of inflammation and demyelination similar to MS.
In rodents such as mice and rats, EAE models can be induced by immunization with spinal cord homogenates, purified myelin, myelin proteins (e.g., myelin basic protein MBP, protein lipoprotein PLP, and myelin oligodendrocyte glycoprotein MOG), or peptides of these proteins. This may be because myelin-specific T cells are activated in the periphery, cross the blood-brain barrier into the central nervous system and are reactivated, triggering a series of inflammatory responses, leading to demyelination and axonal cell apoptosis, ultimately leading to nerve injury and loss of function. Clinical symptoms are assessed using a standardized scoring system, which measures the degree of induction of the disease, with focal demyelination and inflammatory leukocyte infiltration seen in the stained sections of the pathological tissue.
The MOG-induced EAE disease model protocol described here was established in C57BL/6 mice and in B-hIL17A humanized mice developed by Biocytogen, andcan be used for pharmacodynamic evaluation of MS-related drugs.
In vivo small molecule efficacy in C57BL/6 EAE model induced by MOG35-55 emulsion and PTX
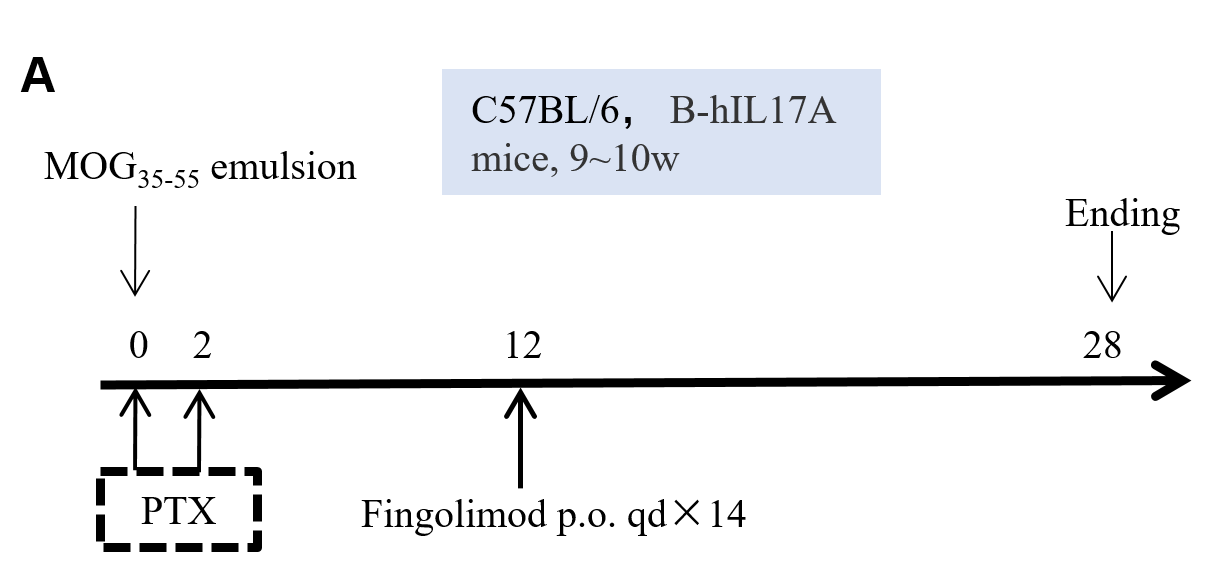
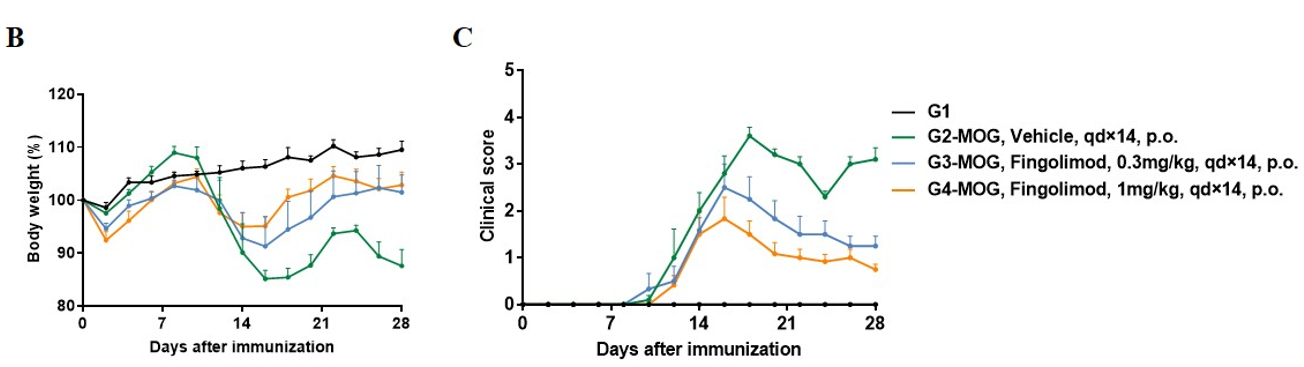
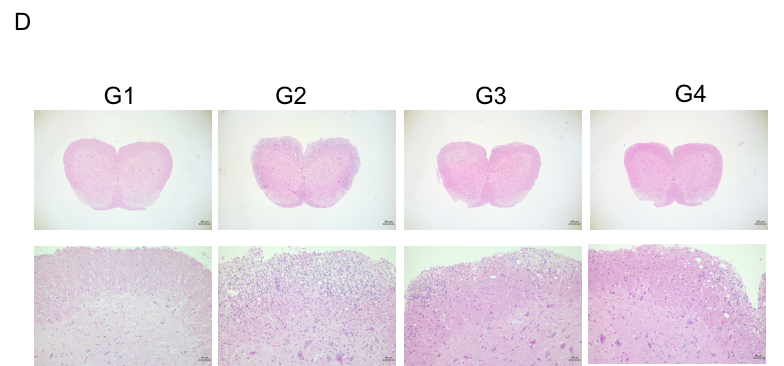
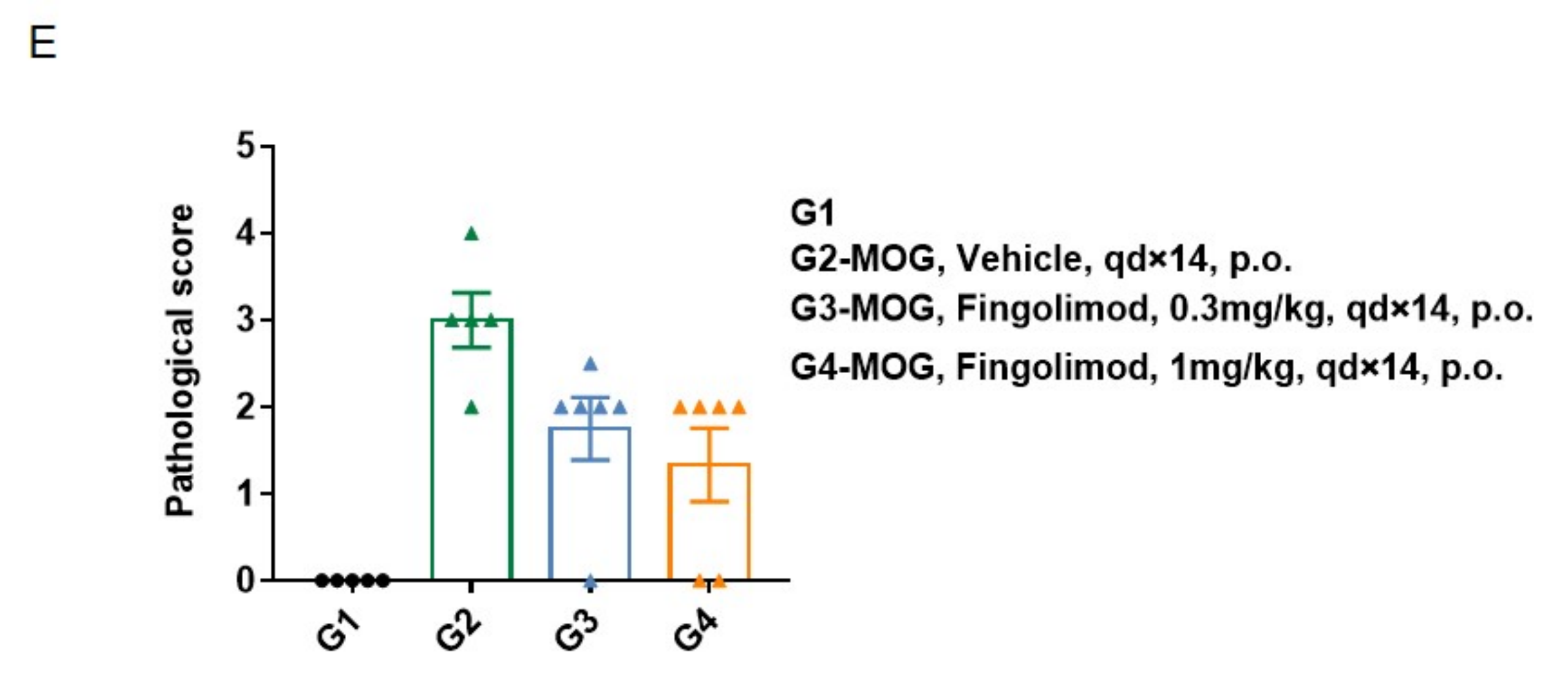
In vivo small molecule efficacy in C57BL/6 EAE model induced by MOG35-55 emulsion and PTX.
(A) Schematic of C57BL/6 EAE model induced by MOG35-55 emulsion. (B) Body weight , and (C) clinical scores were measured in wild-type C57BL/6 mice(n=6, 10 week-old) exposed to control (G1) or MOG35-55 /Vehicle (G2) and MOG35-55 /Fingolimod (G3,G4) as indicated in the dosing regimen. Compared to control (G1), MOG35-55 treated mice (G2-G4) displayed tail weakness, lameness, hind limb paralysis and other symptoms, resulting in a significantly increased clinical score. This data demonstrates successful EAE induction in wild-type C57BL/6 mice. When fingolimod was administered, it was observed that the clinical symptoms slowed down in a dose-dependent manner. Values are expressed as mean ± SEM. MOG35-55: Myelin oligodendrocyte glycoprotein polypeptide 35 and 55. PTX: pertussis toxin.
Product list
|
Product name |
Product number |
|
B-hIL17A mice |
110053 |
|
B-hTNFA mice |
110002 |
References
1. Gaffen, S.L., Jain, R., Garg, A.V. & Cua, D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14, 585-600 (2014).
2. Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J Clin Invest 116, 1218-1222 (2006).
3. Kuwabara, T., Ishikawa, F., Kondo, M. & Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm 2017, 3908061 (2017).






 +86-10-56967680
+86-10-56967680 info@bbctg.com.cn
info@bbctg.com.cn