



Basic Pathological Assay Process
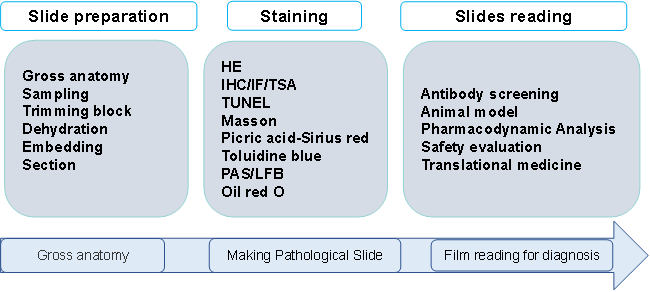
Research Contents
Fully automated high-throughput tissue sectioning and tissue microarray preparation in combination with HE, IHC, IF, multicolor immunofluorescence staining analysis, as well as HALO AI data analysis system; in situ pathological diagnosis, non-clinical safety studies and in situ tissue microenvironment biomarker studies; histopathological analysis of different species and disease models.
Diagnostic Pathology Analysis: Animal models— asthma/ AD/ psoriasis/ IBD/ CIA/ EAE/ PDX/ immune reconstitution
Pathological Analysis Related to Efficacy Evaluation: Scoring methods—qualitative analysis & semi-quantitative analysis: Image J; Halo
Pathological Analysis Related to Toxicity Evaluation: Key points of pathological evaluation: nature and extent of lesions; presence of drug-related organ changes; dose correlation.
Center for Translational Clinical Medicine
Preparation of Tumor Diagnosis/Pathology Report
Diagnosis: HE staining, IHC staining (in combination with other test results)
Report: Case information, medical history, microscopic findings, photographs, diagnostic results, etc.
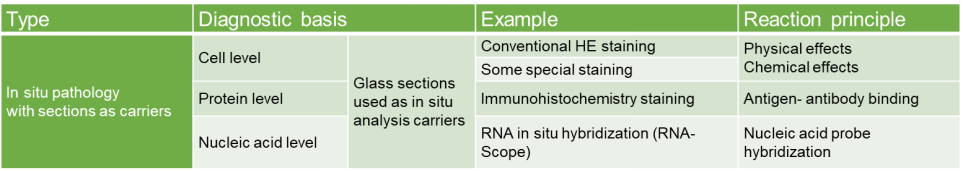
Assay Method
Conventional H&E Staining (Haematoxylin and Eosin Stain)
Normal staining/conventional staining/HE staining. One of the most commonly used methods for paraffin and frozen sections. It facilitates pathological diagnosis while using auxiliary methods to ensure diagnostic accuracy and integrity. The nucleus, cytoplasm, and cartilage are stained blue-black, pink, and blue, respectively.
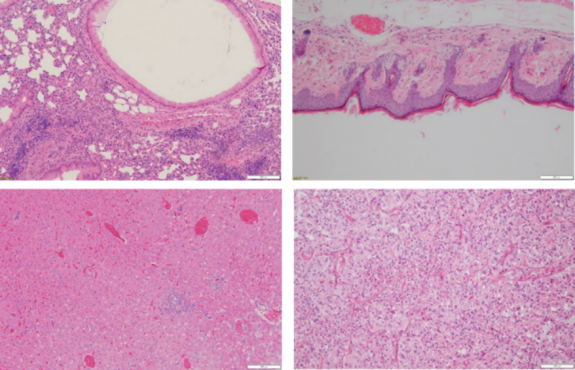
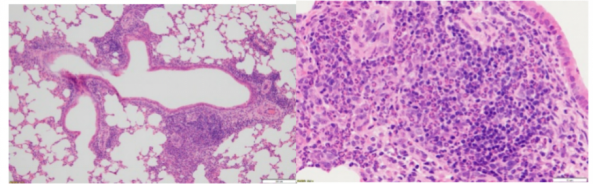
Asthma Model: Inflammatory Cell/Eosinophil Infiltration
HE diagnosis
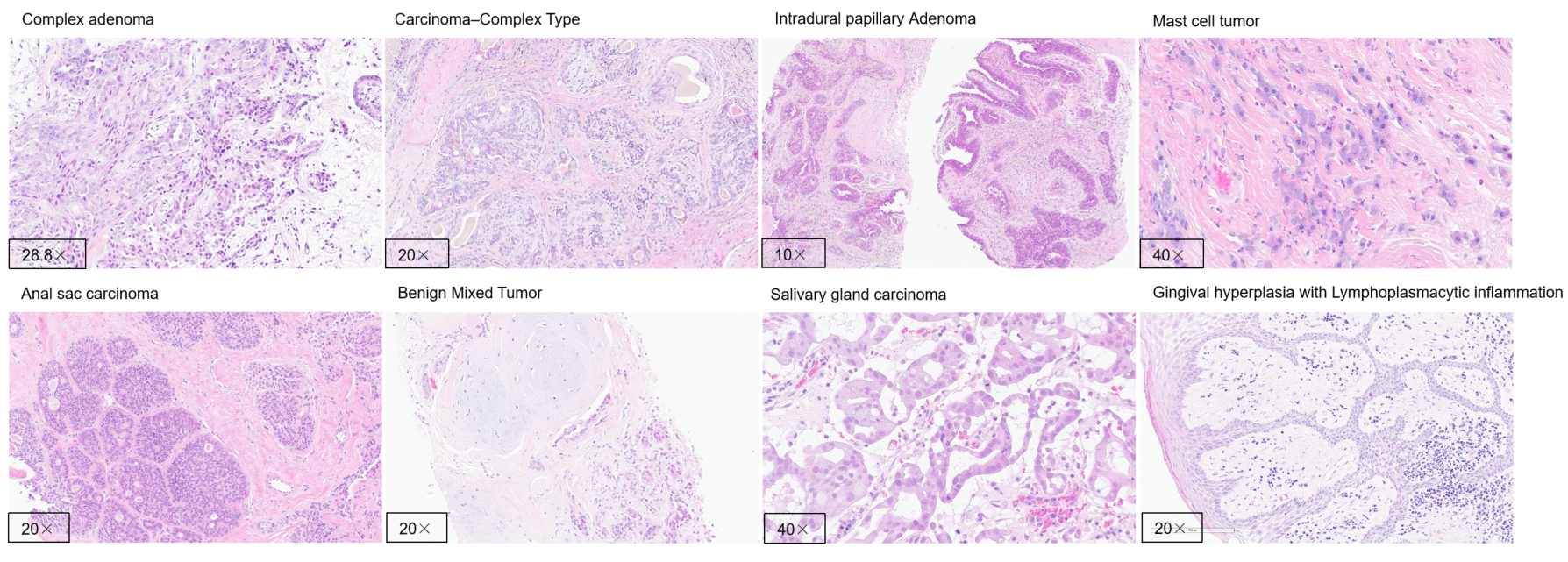
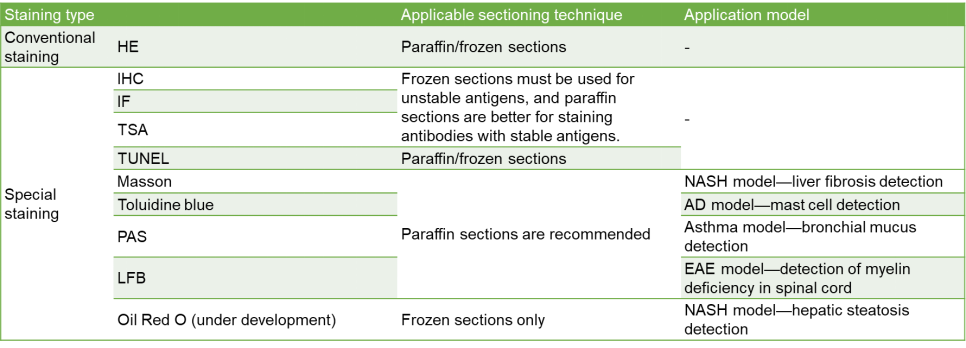
Special Pathological Staining (Example)
IHC Assay
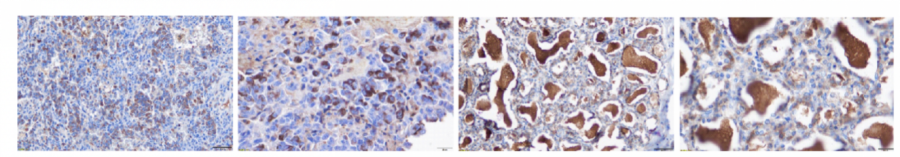
Anti-hPD-L1
Immunofluorescence (IF) Assay
TUNEL Staining (TdT-Mediated dUTP Nick End Labeling Stain): Paraffin & Frozen Sections.
Biotinylate-labeled dUTP can be linked to the 3'-OH termini of DNA breaks in apoptotic cells as catalyzed by terminal deoxynucleotidyl transferase, and binds specifically to streptavidin linked to horseradish peroxidase, and then streptavidin reacts with the POD substrate H2O2 and DAB to produce a dark brown color to specifically and accurately localize apoptotic cells, which can be observed under an optical microscope. Normal or proliferating cells have almost no DNA breaks and will rarely be stained due to the absence of 3'-OH formation. It is a method that integrates molecular biology and morphology, in which in situ staining of intact single apoptotic cell nuclei or apoptotic body can accurately reflect the most typical biochemical and morphological features of apoptosis.
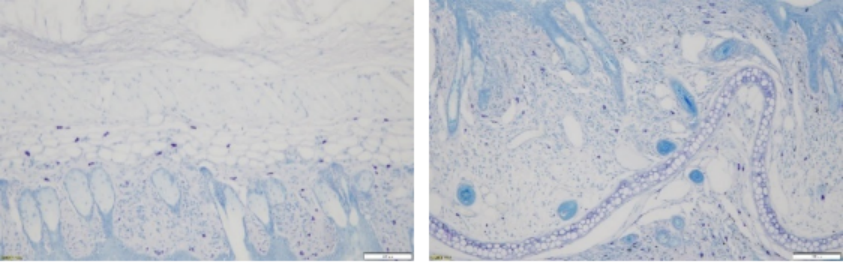
Principle of Toluidine Blue Staining (Paraffin Sections Are Recommended):
Toluidine blue is a blue cationic dye which interacts with acidic tissue components and stains the nucleus blue. In addition, the mast cell cytoplasm contains heparin, histamine, and other heterochromatic substances which are stained metachromatic purplish red with toluidine blue, and hence toluidine blue is used for the initial condyloma acuminatum screening and mast cell detection.
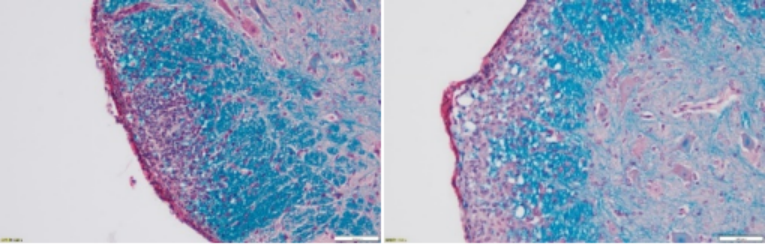
Principle of Masson Staining (Paraffin Sections Are Recommended):
The principle of this staining method is related to the size of the anionic dye molecule and tissue penetration. The size of the molecule is reflected by molecular weight: Compounds with a small molecular weight can easily penetrate densely structured and low-permeable tissues, while compounds with a large molecular weight can only enter loosely structured and highly permeable tissues. However, light green or aniline blue has a large molecular weight, so after Masson staining, muscle fibers are red and collagen fibers are green (light green) or blue (aniline blue), and they are mainly used to distinguish collagen fibers from muscle fibers.
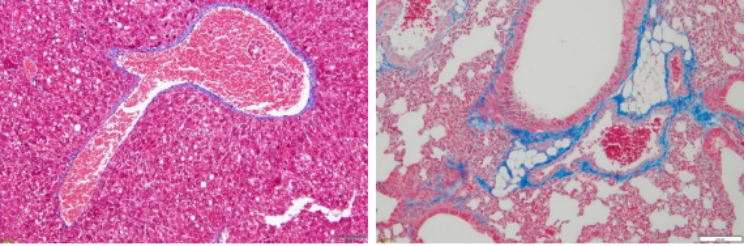
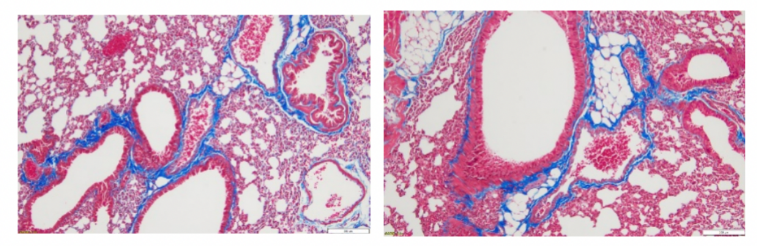
Asthma model: Degree of peribronchial fibrosis
Principle of Periodic Acid-Schiff (PAS) Staining: (Paraffin Sections Are Recommended)
Periodic Acid-Schiff (PAS) staining in histology is mainly used to detect glycogen or other polysaccharide substances in tissues. PAS positive substances are stained red.
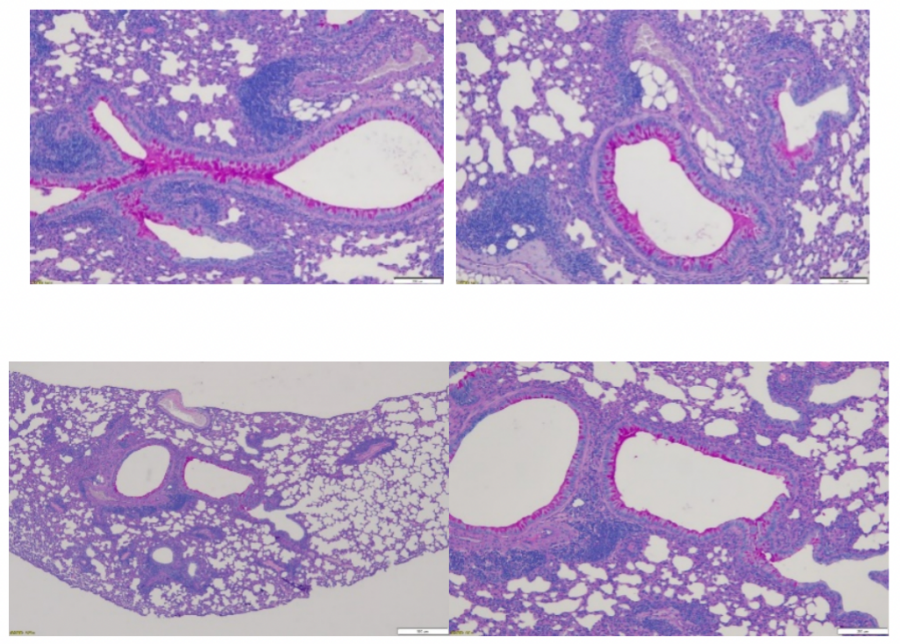
Asthma model: Bronchial mucus assessment
Principle of Luxol Fast Blue (LFB) Staining: (Paraffin Sections Are Recommended)
Luxol Fast Blue (LFB) is a copper phthalocyanine dye capable of binding to lipoproteins of the myelin sheath when dissolved in alcohol. It is used to visualize myelin in nerve tissue.
Generally, the brain, spinal cord, and peripheral nerves are stained. The nerve myelin sheath appears bright blue with a colorless to light blue background, which is mainly used to show the morphological structure and pathological changes of nerve myelin sheath.
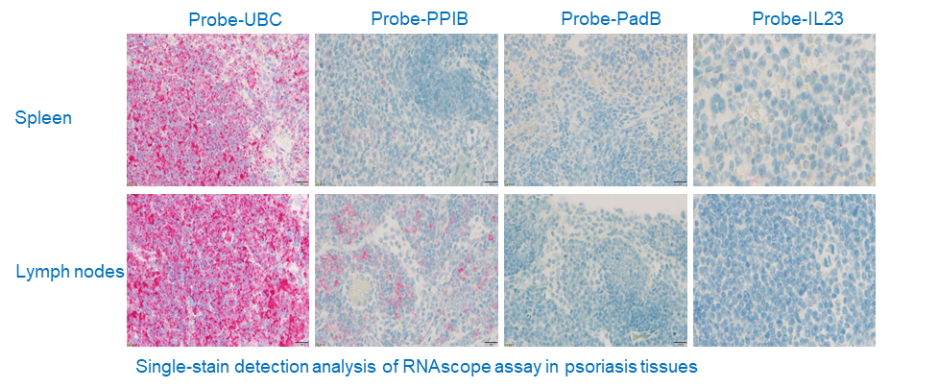
RNAscope
It is a novel RNA ISH technology with a unique probe design strategy that allows simultaneous signal amplification and background suppression to achieve single-molecule visualization while preserving tissue morphology.
Principle: Tissue sections (FFPE) are first properly pretreated and then the target RNA is hybridized with specific oligonucleotide probes with ACD proprietary. The RNAscope® 2.5 HD Duplex Chromogenic Assay employs two independent signal amplification systems, each using a different chromogenic enzyme to react with substrate for color development. Two target RNA transcripts are shown in two different colors as dots or clustered signals which are visible at 40–100× magnification under bright field microscope.
A visual process of the target RNA hybridization with the probe, followed by signal amplification and then color development.
Compensate the shortcoming of IHC assay
Easily degradable or secretory proteins
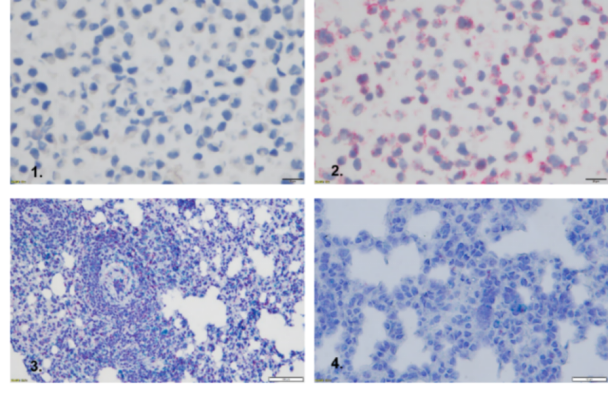
1.Negative control
2.Housekeeping gene PPIP (positive control)
3.Housekeeping gene (positive control)
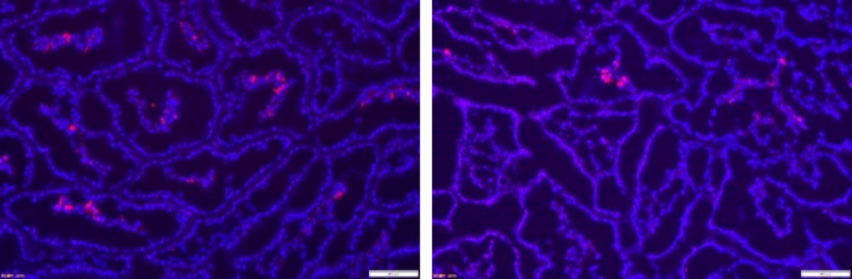
4.IL4/IL13 double staining (red and azure)
Tissue Microarrays (TMAs)
Tissue microarrays (TMAs), also known as tissue chips, arrange tens, hundreds, or even thousands of small tissues neatly on a carrier.
Advantages:
High throughput, massive information acquisition, and significantly higher efficiency;
Consistent experimental conditions for less experimental errors;
Substantial reduction in research costs;
High comparability and accuracy;
Optimal storage conditions for original paraffin blocks;
Convenience in establishing various experimental controls;


Preclinical toxicology studies are largely focused on drug safety evaluation. Whether a drug is safe and effective is the determining factor in drug development, and toxicity (safety) is a major cause for study discontinuation throughout the whole drug development process. The aim is to provide a reliable toxicokinetic basis for clinical (human) trials by studying the relationship between drug exposure and toxic response in animal models, explaining possible target organs of toxicity and toxic responses, and predicting drug safety in humans.
Histopathological Analysis
Inflammation and Cytokine Storm Assays
As part of the immune response, inflammation plays an important role in defending the body against pathogens, such as viruses, bacteria, fungi, and other parasites. However, the inappropriate activation of inflammatory processes is an underlying contributor to many common pathological conditions. For example, autoimmune conditions arise when our immune system mistakes our cells or tissues for pathogens and attacks them. In addition, studies show that tumor proliferation and metastasis may occur when inflammatory cytokines create a microenvironment conducive to cancer progression.
The overall effect of an inflammatory response is dictated by the balance between pro- and anti-inflammatory mediators. Pro-inflammatory cytokines (such as IL-1 β, IL-6, and TNF α) are responsible for early responses and amplification of inflammatory reactions, whereas anti-inflammatory cytokines (including IL-4, IL-10, and IL-13) have the opposite effect in that they limit the inflammatory reactions. The increasing complexity of pro- and anti-inflammatory cytokine and chemokine networks has made it crucial to examine them in relevant functional groups rather than individually.
The so-called cytokine storm or cytokine release syndrome (CRS) is characterized by an aggressive pro-inflammatory response in combination with an insufficient anti-inflammatory response, which results in the loss of homeostasis of the immune response. The key factors identified in the pathology of a cytokine storm are TNF α, interferons, IL-1β, MCP-1 (CCL2) and, most importantly, IL-6.
|
Target |
Type |
|
G-CSF (CSF-3) |
Cytokine (colony-stimulating factors) |
|
GM-CSF |
Cytokine (colony-stimulating factors) |
|
IFN α |
Cytokine (interferon), pro-inflammatory |
|
IFN γ |
Cytokine (interferon), pro-inflammatory |
|
IL-1 β |
Cytokine, pro-inflammatory |
|
IL-2 |
Cytokine, pro-inflammatory |
|
IL-4 |
Cytokine, anti-inflammatory |
|
IL-5 |
Cytokine |
|
IL-6 |
Cytokine, pro-inflammatory |
|
IL-8 (CXCL8) |
Chemokine (CXC type), pro-inflammatory |
|
IL-10 |
Cytokine, anti-inflammatory |
|
IL-12p70 |
Cytokine, pro-inflammatory |
|
IL-13 |
Cytokine, immunoregulatory |
|
IL-17A (CTLA-8) |
Cytokine, pro-inflammatory |
|
IL-18 |
Cytokine, pro-inflammatory |
|
IP-10 (CXCL10) |
Chemokine (CXC type) |
|
MCP-1 (CCL2) |
Chemokine (CC type) |
|
MIP-1 α (CCL3) |
Chemokine (CC type) |
|
MIP-1 β (CCL4) |
Chemokine (CC type) |
|
TNF α |
Tumor necrosis factor |
|
TNF β |
Tumor necrosis factor |
ELISA
Principle: The antigen and antibody bind to form a solid phase immune complex, and the enzyme-labeled antibody is added to react with the substrate for qualitative or quantitative analysis of antigen/antibody according to the degree of color development.
Method:
Double antibody sandwich (a common method for antigen detection): A solid-phase carrier is coated with specific antibodies to detect the antigen in the test sample.
Cytometric Bead Array (CBA)
Cytometric Bead Array (CBA) is a flow cytometry application that allows users to quantify multiple proteins simultaneously. It integrates various features such as immune beads, laser detection, signal processing, and computational calculation, and is capable of qualitative and quantitative analysis of multiple indicators in a sample. Compared with ELISA, the assay features higher analyte capture efficiency, faster processes, and more accurate results, while using fewer samples.

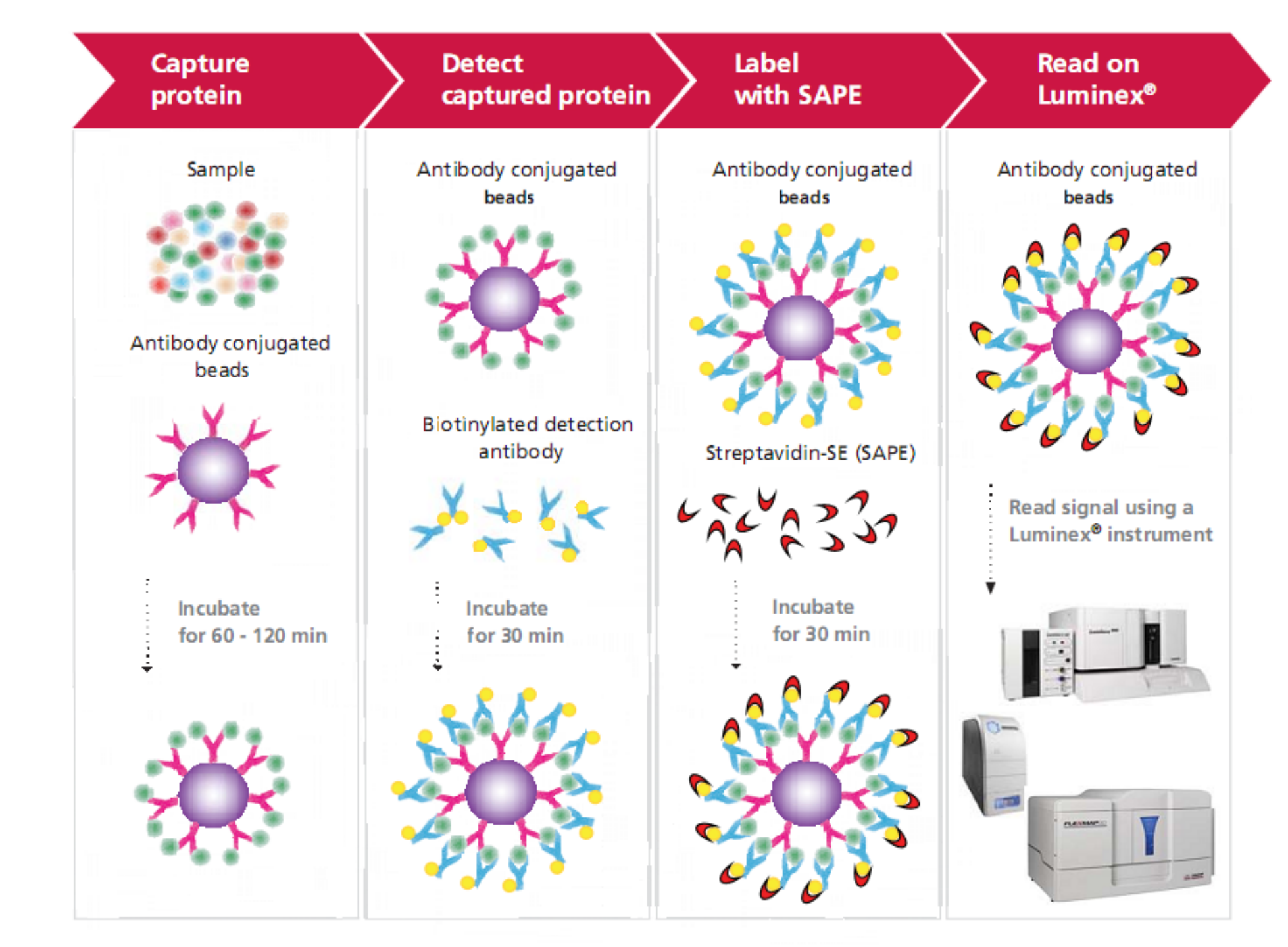
Luminex Multiplex Platform
Using a smaller sample size, the Luminex® xMAP technology can simultaneously detect and quantify up to 500 factors, including cytokines, chemokines, growth factors, metabolic, endocrine, and neurological indicators, antibody subtypes, and molecules related to tumors and signaling pathways. The technology employs differentially stained capture beads for each target in a multiplex "ELISA-like" assay. Compared with traditional techniques (such as ELISA) for quantitative detection of liquid-phase sample indicators, the technology allows quantitative and qualitative detection of dozens or hundreds of analytes using significantly less sample at the same time.
Hematology Assay
Routine Blood Test
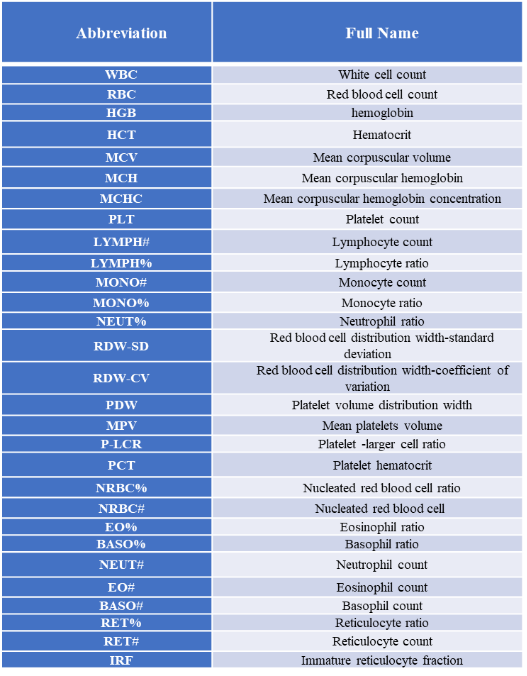
Routine blood tests help determine blood conditions and diagnose diseases by observing the changes in the number and morphological distribution of blood cells. The tests can be completed by machines using modern automated technologies. Routine blood tests include red blood cell count (RBC), hemoglobin (Hb), white blood cell count (WBC), differential white blood cell count and platelets (PLT), which can usually be divided into three major systems, namely the red blood cell system, white blood cell system, and platelet system.
Detectable Indicators:
Blood Chemistry
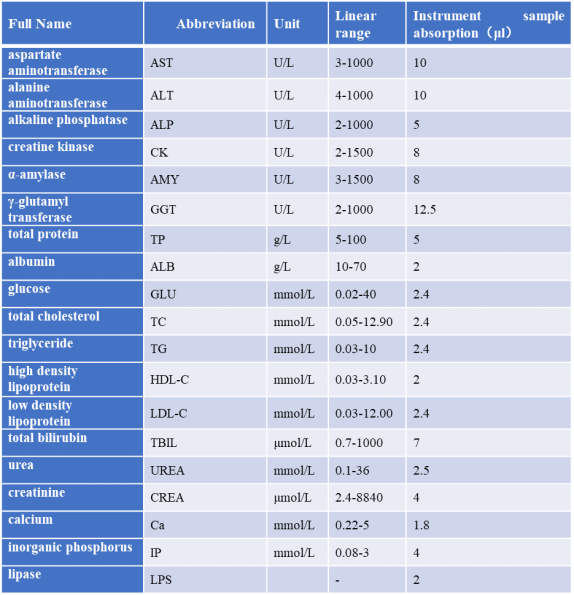
Blood is a bright red liquid that fills the vessels of the body, containing many different substances in addition to blood cells. Blood chemistry tests measure the contents of various ions, sugars, lipids, proteins, as well as various enzymes, hormones, and metabolites present in the blood. They can provide a basis for diagnosis and treatment, assisting in the determination of clinical conditions and tracking treatment efficacy.
Detectable Indicators:
Case study:toxicity evaluation of urelumab in B-h4-1BB mice
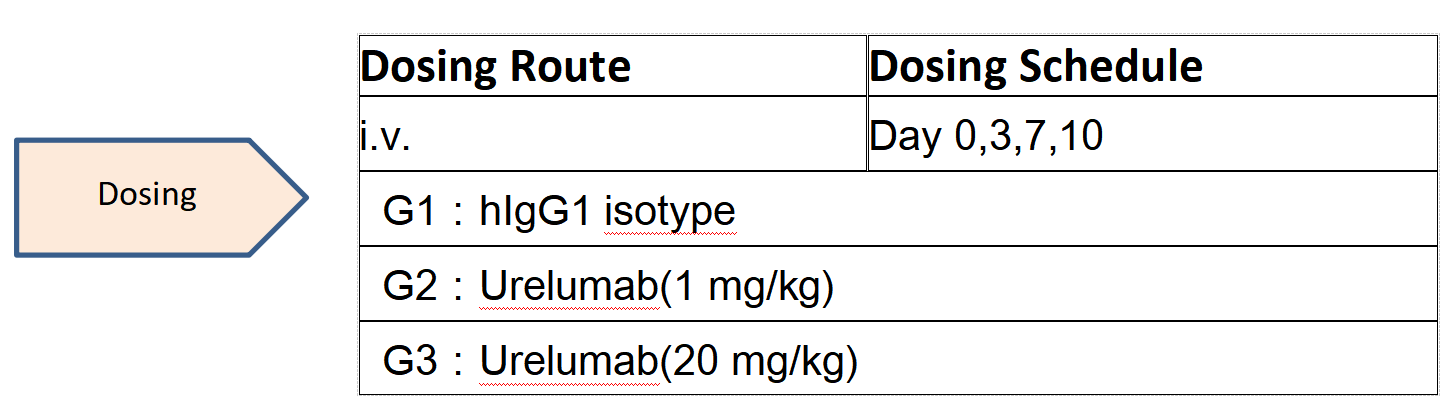
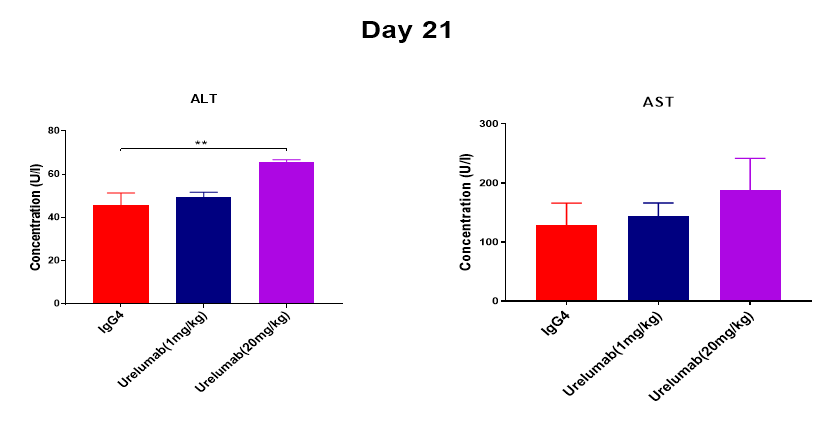
An increase in serum transaminase ALT was found at day 21 after 4 consecutive high doses of Urelumab analogue at 20 mg/kg in humanized 4-1BB mice.






 +86-10-56967680
+86-10-56967680 info@bbctg.com.cn
info@bbctg.com.cn