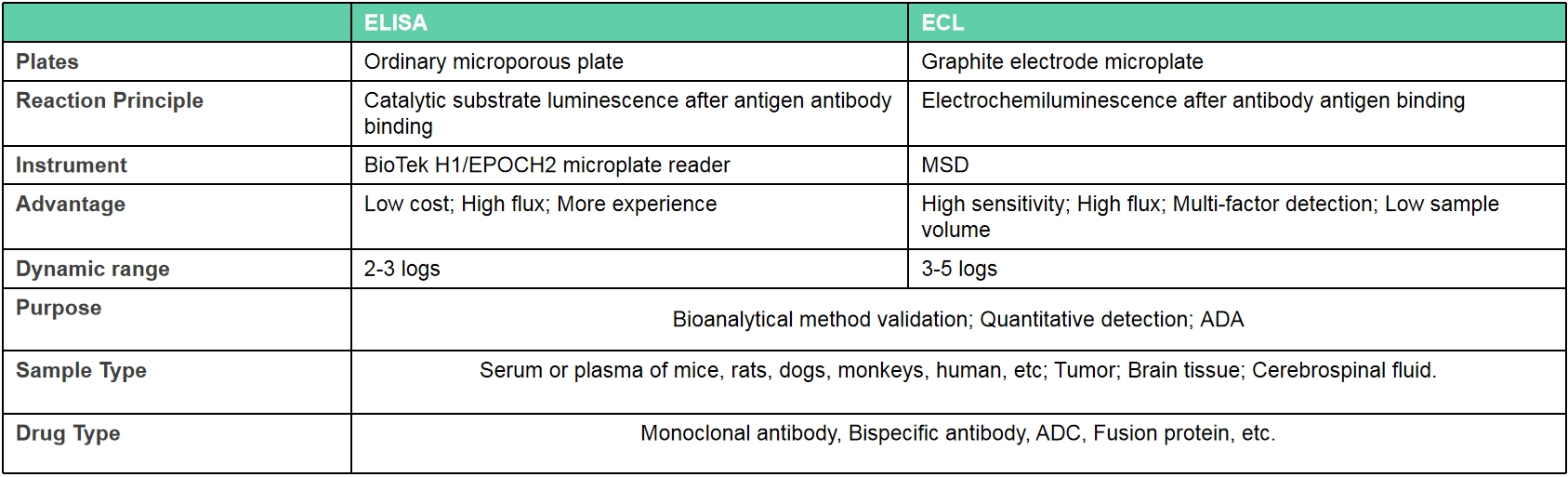
Service profile: Biocytogen has rich experience in preclinical pharmacokinetic testing and bioanalysis, and could provide professional pharmacokinetic analysis services based on antibody drugs. PK bioanalysis platform include ELISA and ECL.
Although it is quite different from traditional chemical drugs, antibody pharmacokinetic research is still based on classical pharmacokinetic research methods, but more attention is paid to its targeting and the pharmacokinetic characteristics affected by antibody characteristics. We have two kinds of experimental types include antibody serum/plasma concentration detection and anti-drug antibody (ADA) screening test.



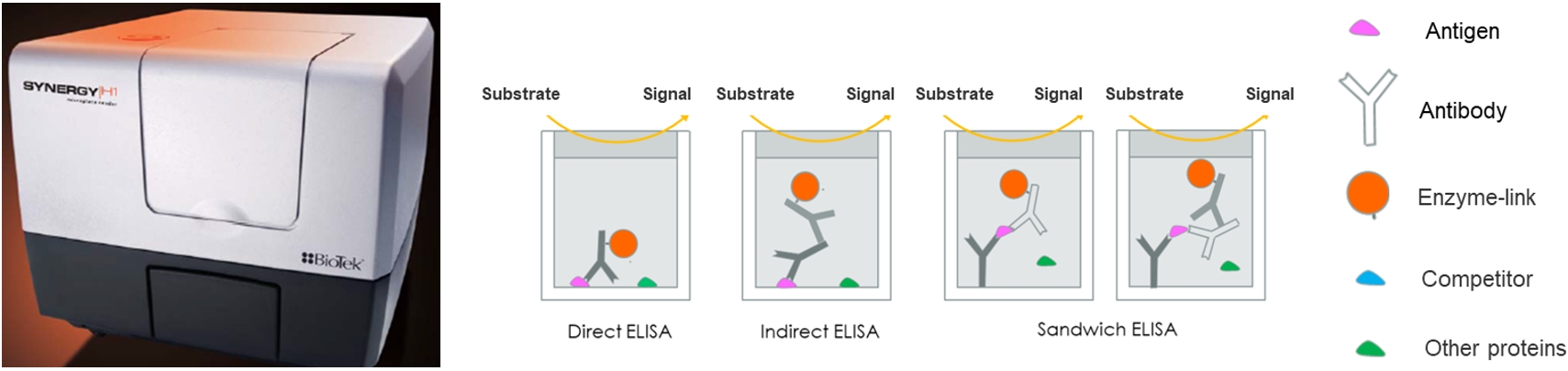
ELISA

Electrochemiluminescence (ECL)
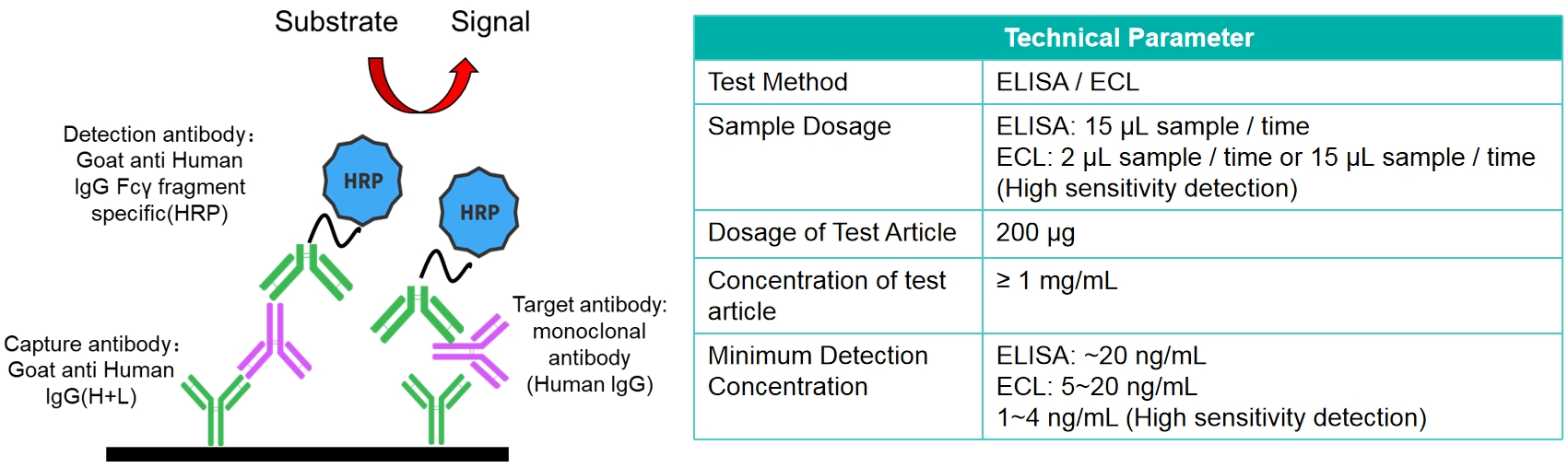
General Assay
The general assay is designed for the detection of hIgG antibody concentration.
Sandwich technique detection based on species specificity
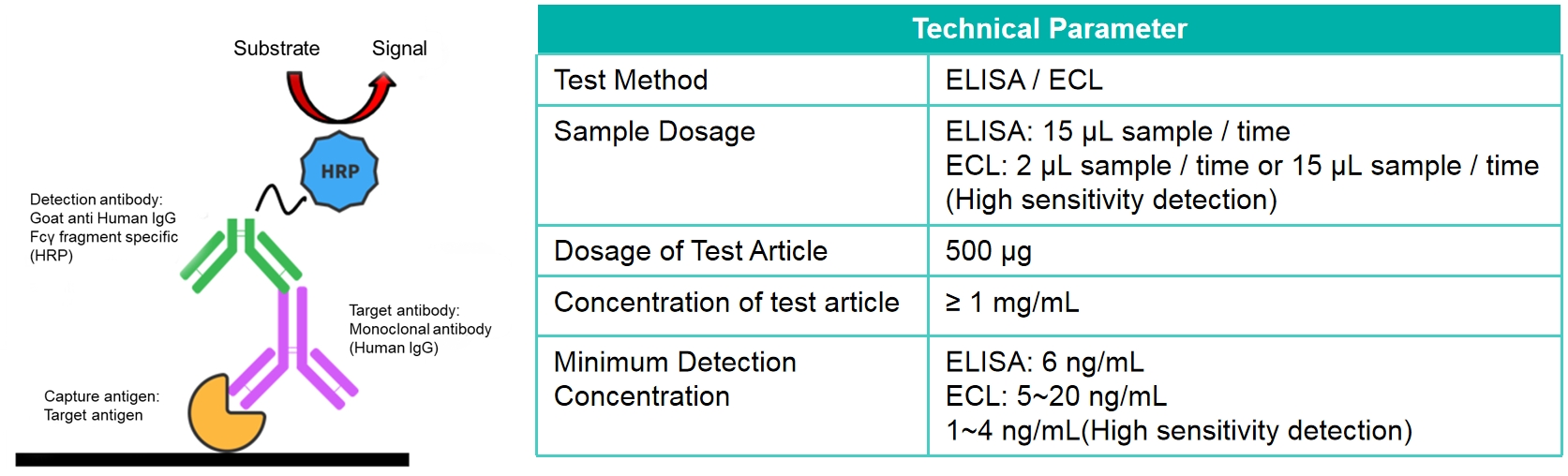
Specific Assay
Detection according to drug target
Bispecific antibody detection
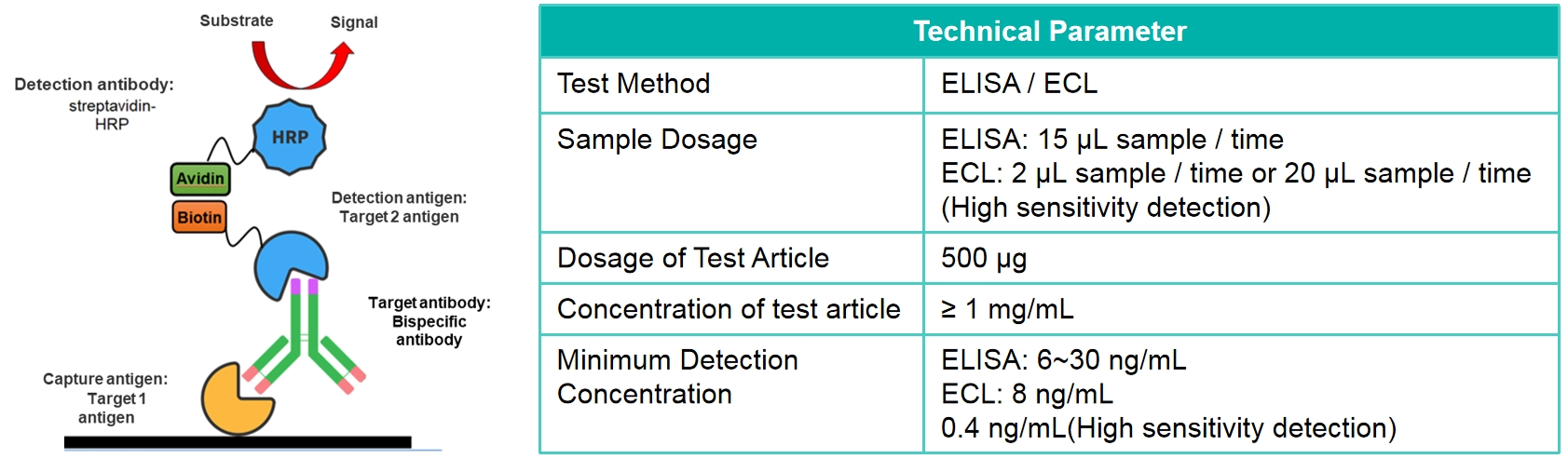
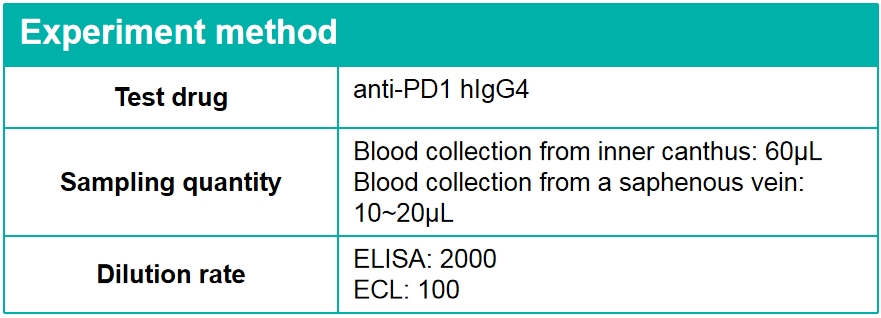
Strategy for ELISA-Alternate blood collection (For T1/2 of antibody is 5~10day)
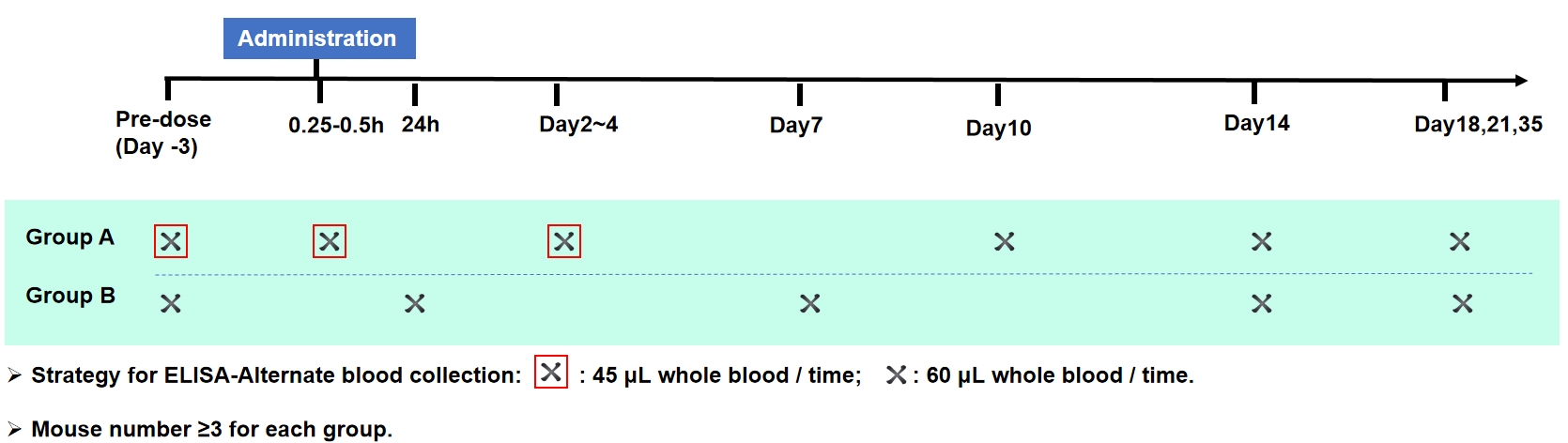
Strategy for ECL-Microsampling(For T1/2 of antibody is 5~10day)
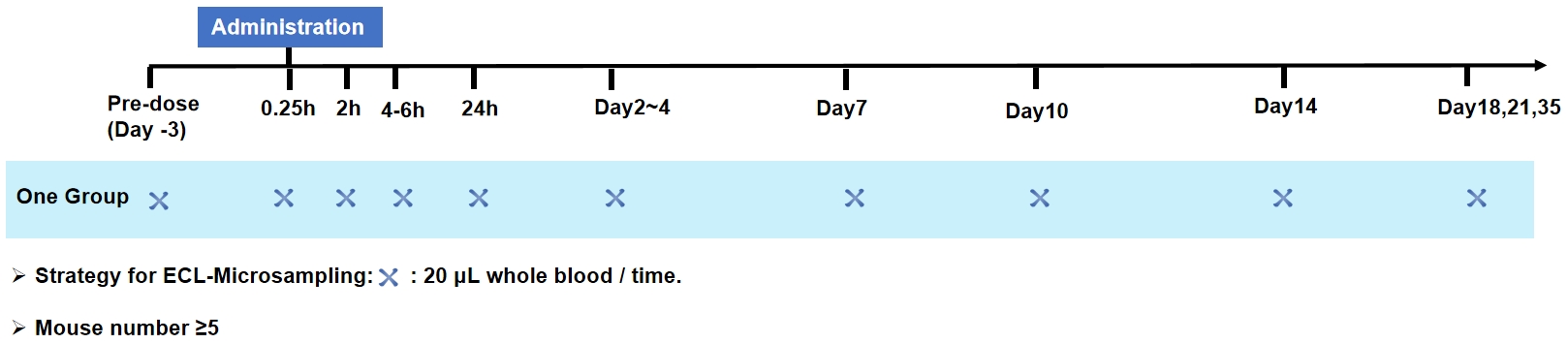
Data analysis
Study design for PK study
- Serum is suggested for PK detection.
- Heparin sodium sometimes influences the results. Anti-coagulants such as EDTA.2Na and EDTA.2K are recommended for plasma collection.
- The administration method consistent with the clinical practice is suggested.
- Intravenous administration is suggested.
- 9-10 week old mice are recommended.
Comparison of sampling method
Comparison between sampling methods in detection results
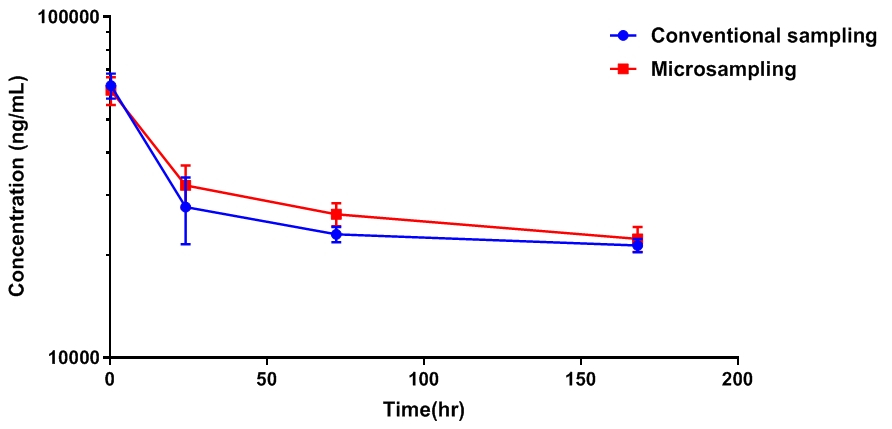
The data show that there is no obvious difference between the experimental results of the two sampling methods.
Correlation of half-lives of Abs between human, monkey, B-hFcRn and WT mice
Table 1. Half-lives (day) of Abs in human, monkey, B-hFcRn and wild-type mice
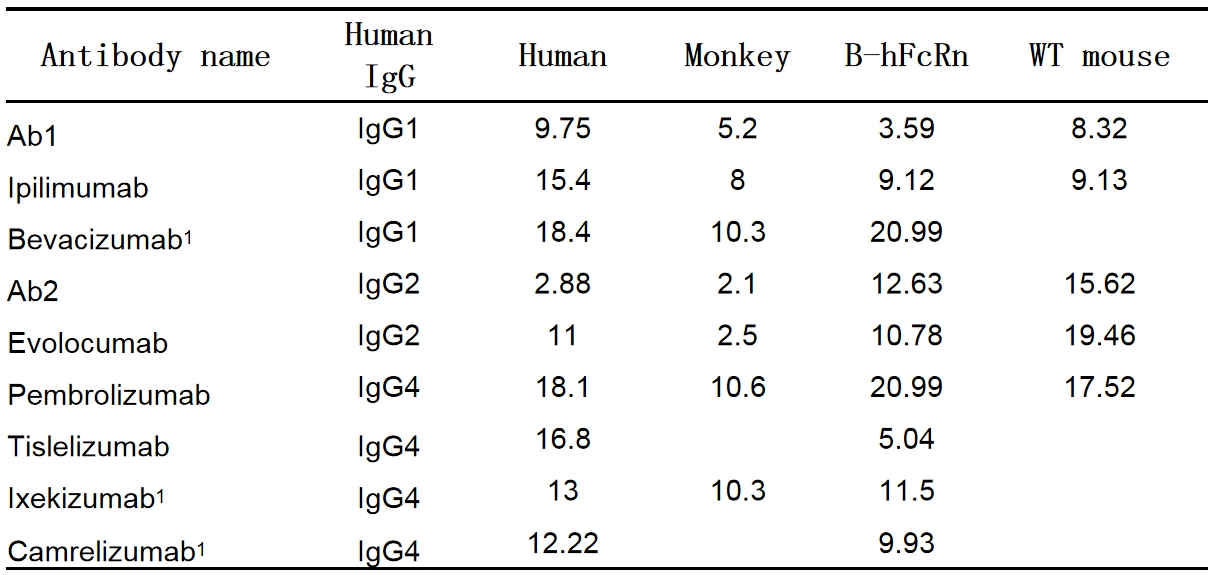
1 The number of bevacizumab samples was relatively small.
Figure 1. Correlations of half-lives between human and monkey (A), B-hFcRn mice (B) (only including human IgG1 mAbs)
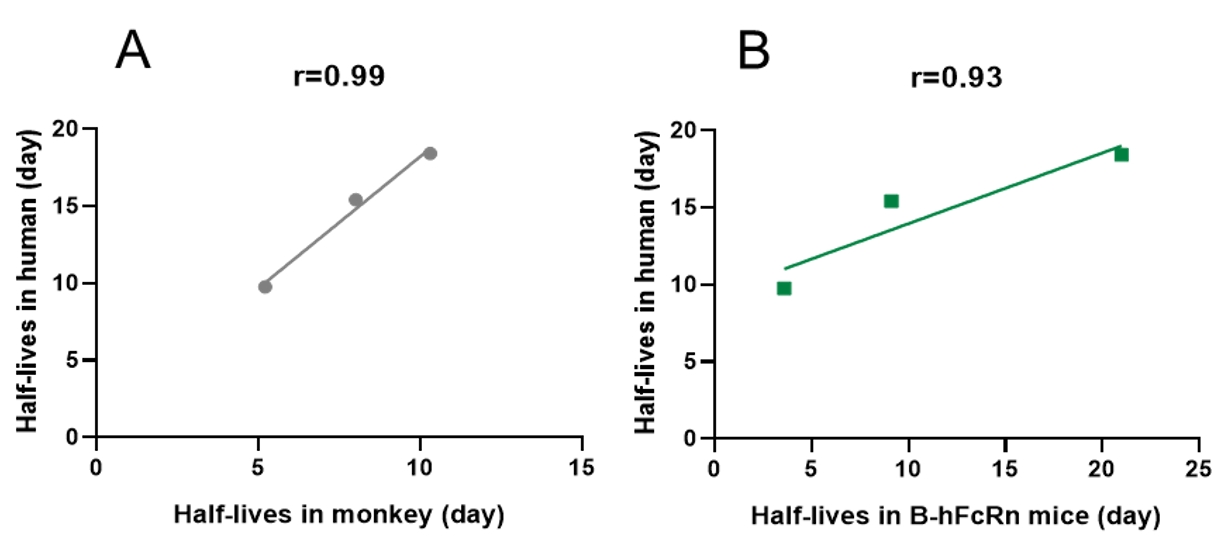
* More abs are being tested.
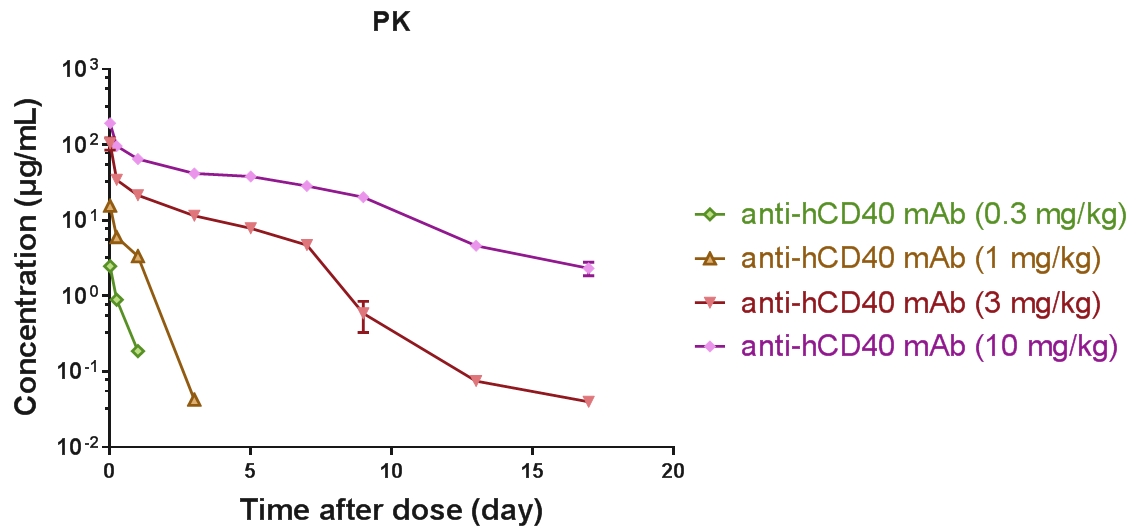
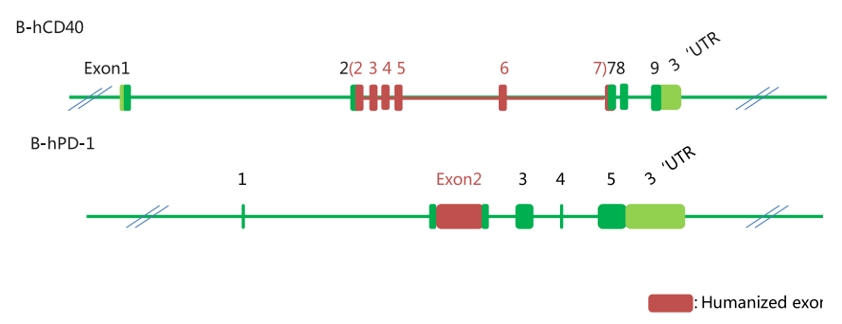
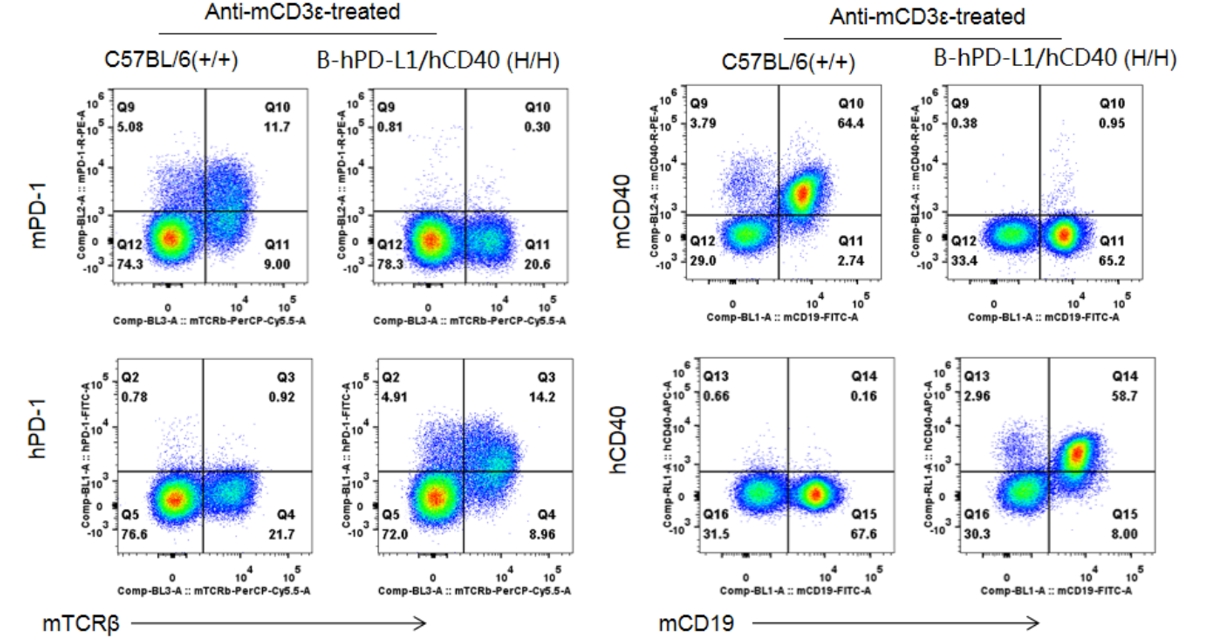
PK/PD/Efficacy of anti-hCD40 antibodies can be tested B-hCD40 mice
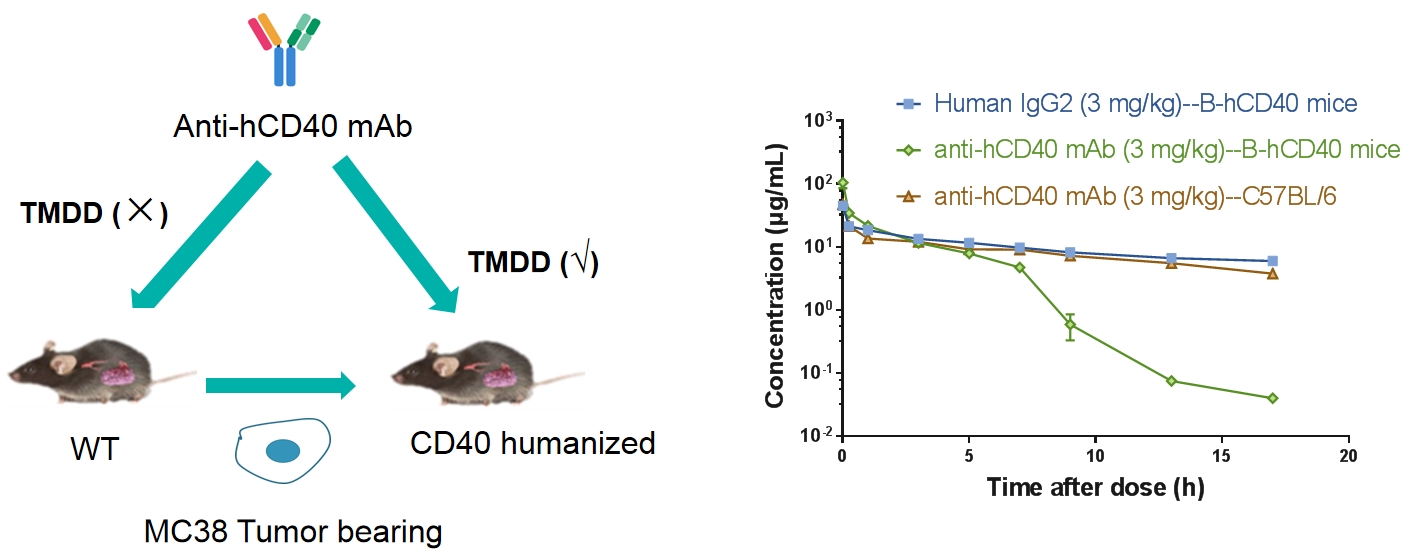
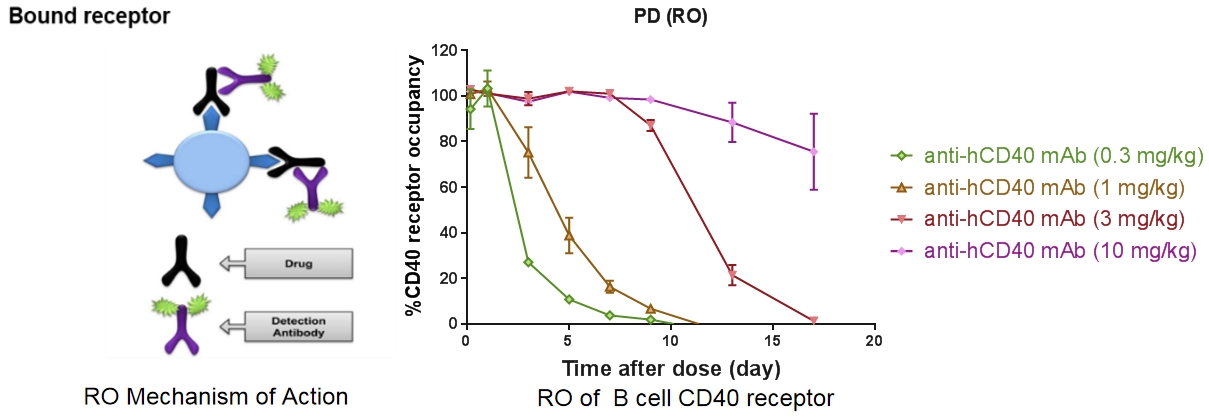
Receptor Occupancy Analysis
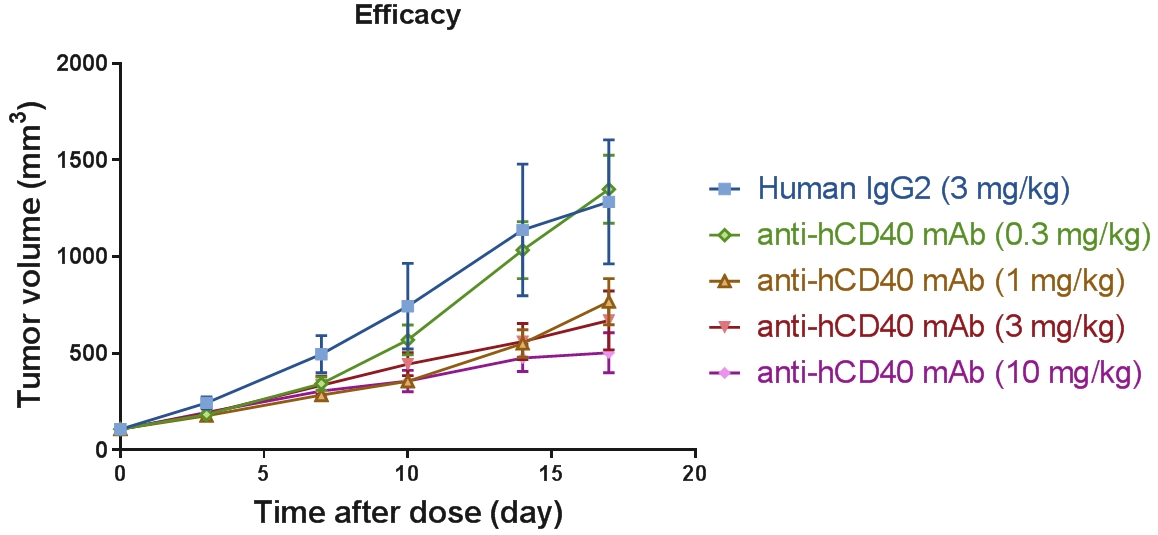
In vivo pharmacodynamic evaluation
Penetration cross blood brain barrier of anti-hTFR1 HCAb
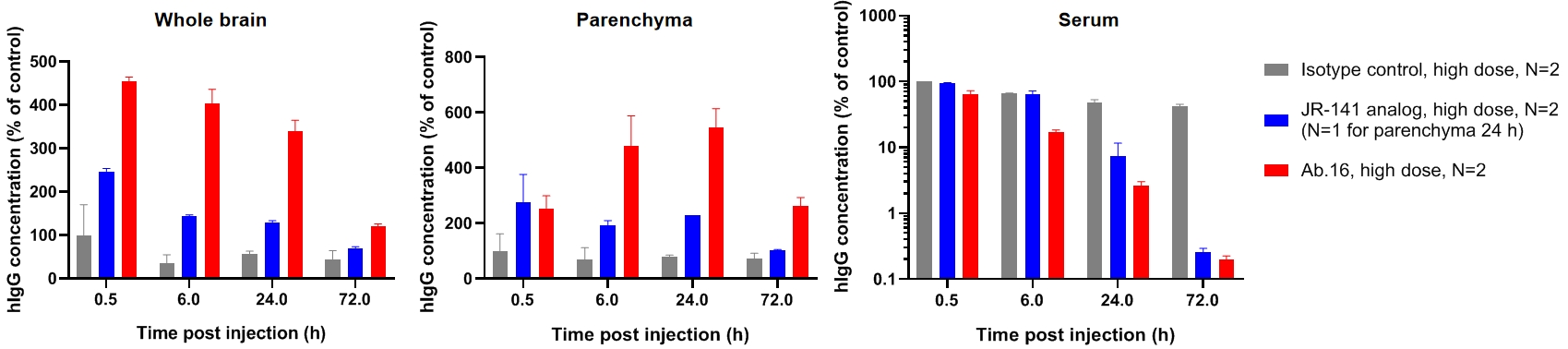
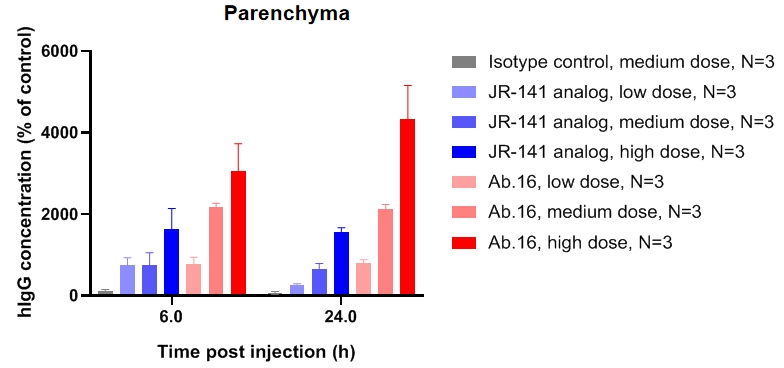
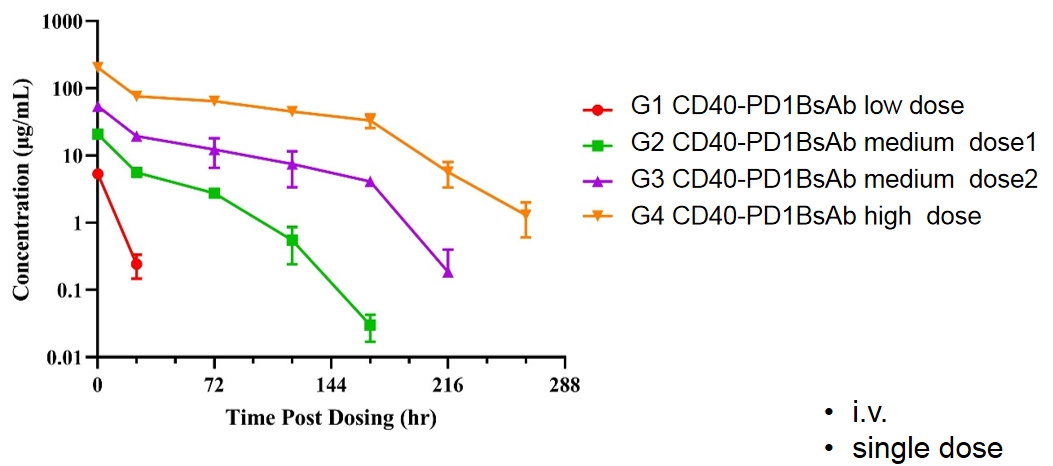
CD40-PD1BsAb
B-hPD1/hCD40 mice
PK profile of CD40-PD1BsAb inB-hPD1/hCD40 mice
HER2×TROP2 BsADC Showed Effective MMAE Accumulation in Tumors with a Great Stability in Circulation From a CDX Model
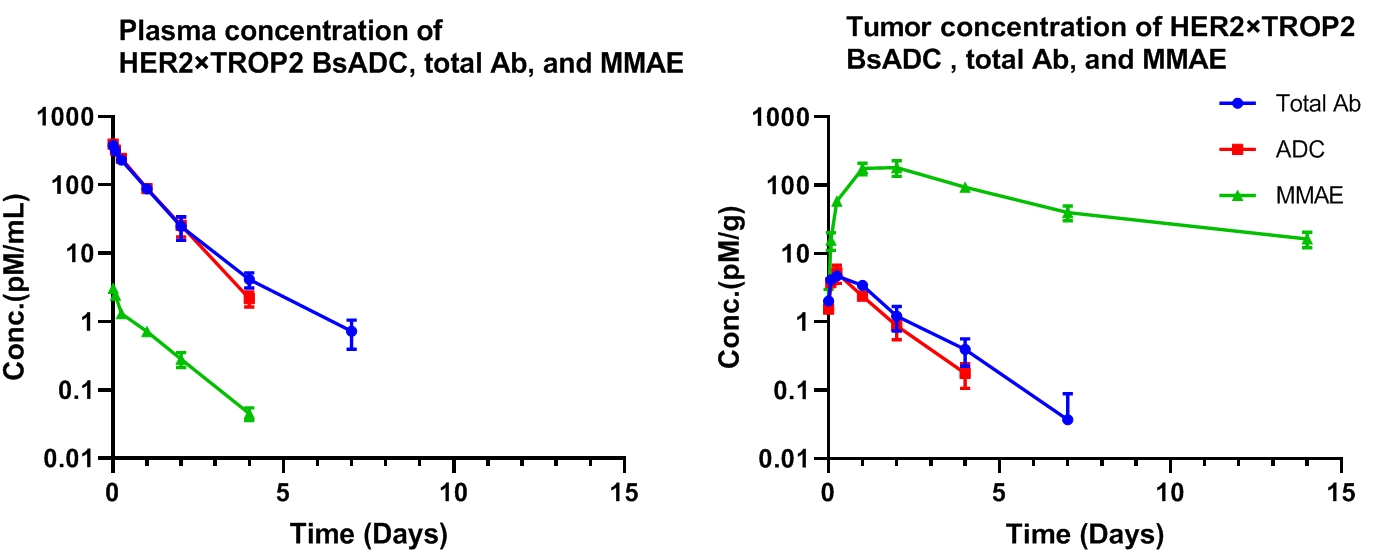
Pharmacokinetic analysis of HER2×TROP2 BsADC in NCI-H1975 xenograft model. After a single dose of 3 mg/kg of HER2×TROP2 BsADC , the payload MMAE was found to accumulate in the tumor but was present at low levels in the plasma. This suggests that HER2×TROP2 BsADC has reached an efficient tumor-targeted delivery of MMAE and may have better antitumor activity.
In the process of using biological products for treatment, some organisms will produce immune responses, the most significant of which is anti-drug antibodies (ADA). Immunogenicity is the ability of a special substance to cause immune responses. The formation of ADA-mAb immune complex is an additional clearance pathway for monoclonal antibodies, which has an important impact on the treatment and elimination of monoclonal antibodies in the body.
The commonly used ADA detection method uses the characteristics of the specific combination of ADA and drugs to detect the anti-drug antibody (essentially mouse anti-human IgG) produced in mice.
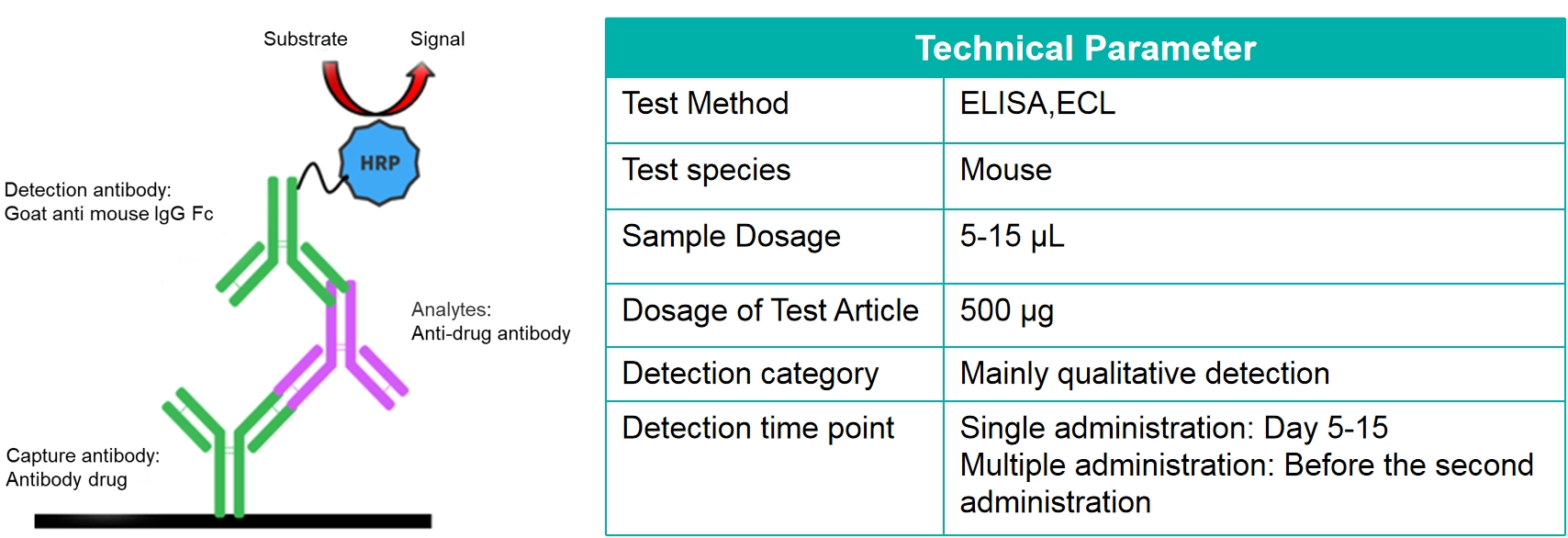
Study case—Detection of ADA in mouse serum
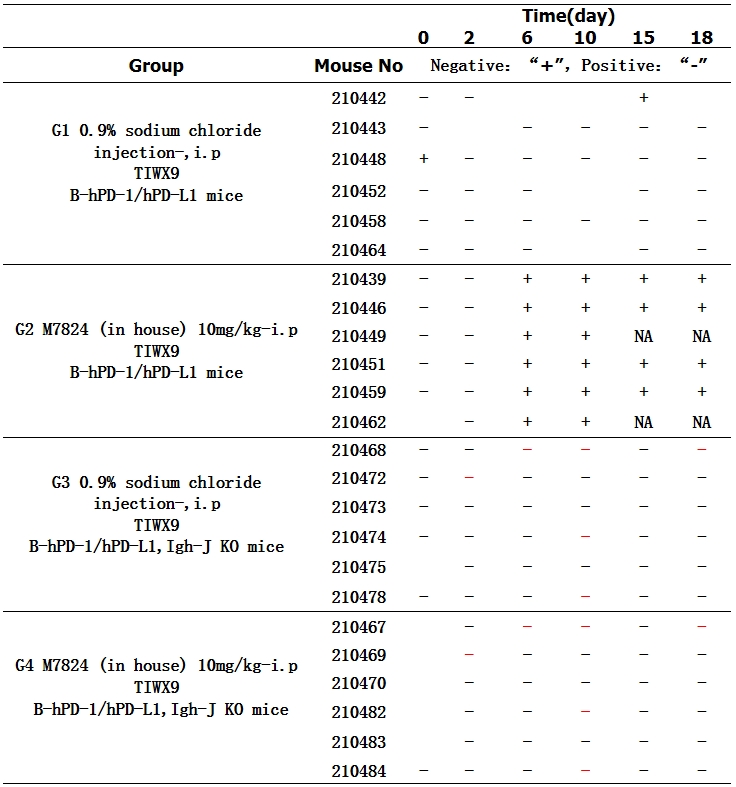
The SNR value (SNR= signal value of a single sample /pool series signal value) was calculated from the samples in the normal saline group and before administration. After excluding the abnormal value, the SNR of the remaining samples was used to calculate the screening threshold value of 1.24. The SNR values of all samples were compared with the screening threshold value, and those higher than the screening threshold value were recorded as positive "+" and those lower than the screening threshold value "-“. NA: unable to sample due to mouse death.
References
1. Guidelines for the survival bleeding of mice and rats.(2010).
2. Diehl K. H. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J.Appl.Toxicol.21,15-23(2001).
3. Janet H, et al. Methods of Blood Collection in the Mouse.Lab Animal.29 (10):47-53.(2010).
4. Bioanalytical Method Validation Guidance for Industry (FDA 2018)






 +86-10-56967680
+86-10-56967680 info@bbctg.com.cn
info@bbctg.com.cn