Introduction
Cell therapy is the transfer of intact, live cells into a patient to help lessen or cure a disease. The cells may originate from the patient (autologous cells) or a donor (allogeneic cells). The cells used in cell therapy can be classified by their potential to transform into different cell types. Pluripotent cells can transform into any cell type in the body and multipotent cells can transform into other cell types, but their repertoire is more limited than that of pluripotent cells. Cells that rapidly reproduce in the body such as immune cells, blood cells or skin cells can usually do so ex vivo given the right conditions. This allows differentiated, adult immune cells to be used for cell therapy. The cells can be removed from the body, isolated from a mixed cell population, modified and then expanded before return to the body. A recently developed cell therapy involves the transfer of adult self-renewing T lymphocytes which are genetically modified to increase their immune potency to kill disease-causing cells. Differentiated or primary cells are of a fixed type and the type of cells administered depends on the treatment.
Recent years, immune cell therapies have got a lot of attention and made great achievements on tumor treatment, especially Car-T therapy on hematological tumor treatment. CAR-T cell stands for chimeric antigen receptor (CAR) T cell therapy. This a way of modifying the patient’s own immune cells (T-cells) to express a receptor on their surface that recognizes structures (antigens) on the surface of malignant cells. Once the receptor binds to a tumor antigen, the T-cell is stimulated to attack the malignant cells. Beyond that, an increasing number of immune cell therapy shows great promise for malignant tumor treatment.
The pharmacology department of Biocytogen have established a state-of-the-art pharmacology platform to study the mechanism of action and the functional effects of various cell therapies, including CAR-T, CAR-Macrophages, CAR-NK, TILs, TCR-T.
Overall Capabilities
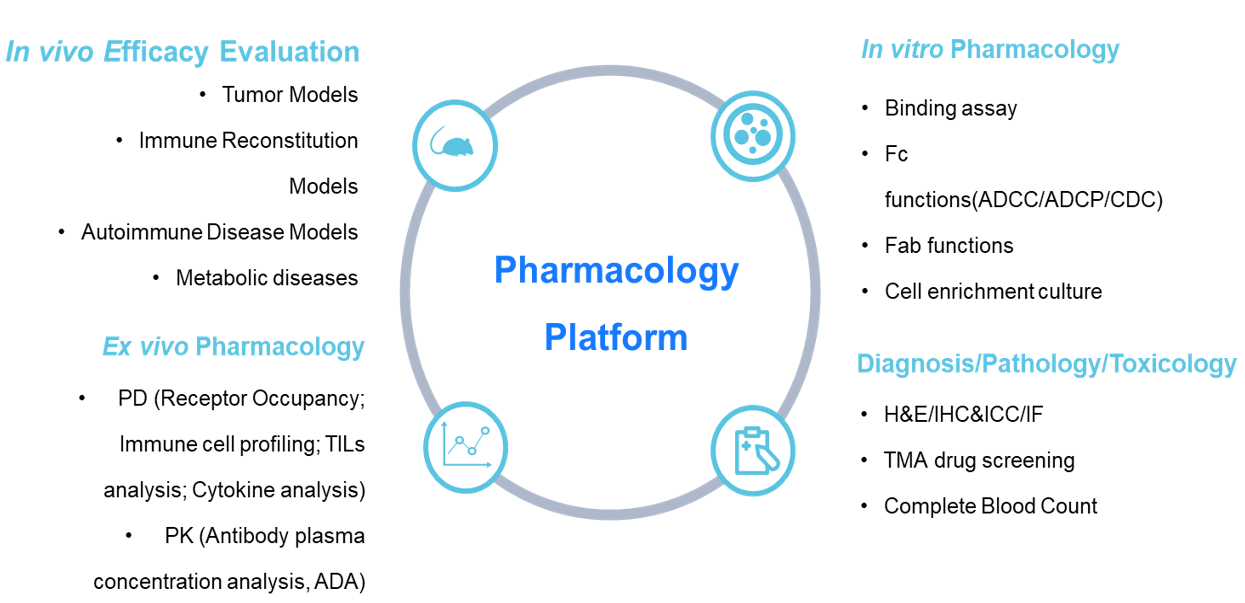
Results
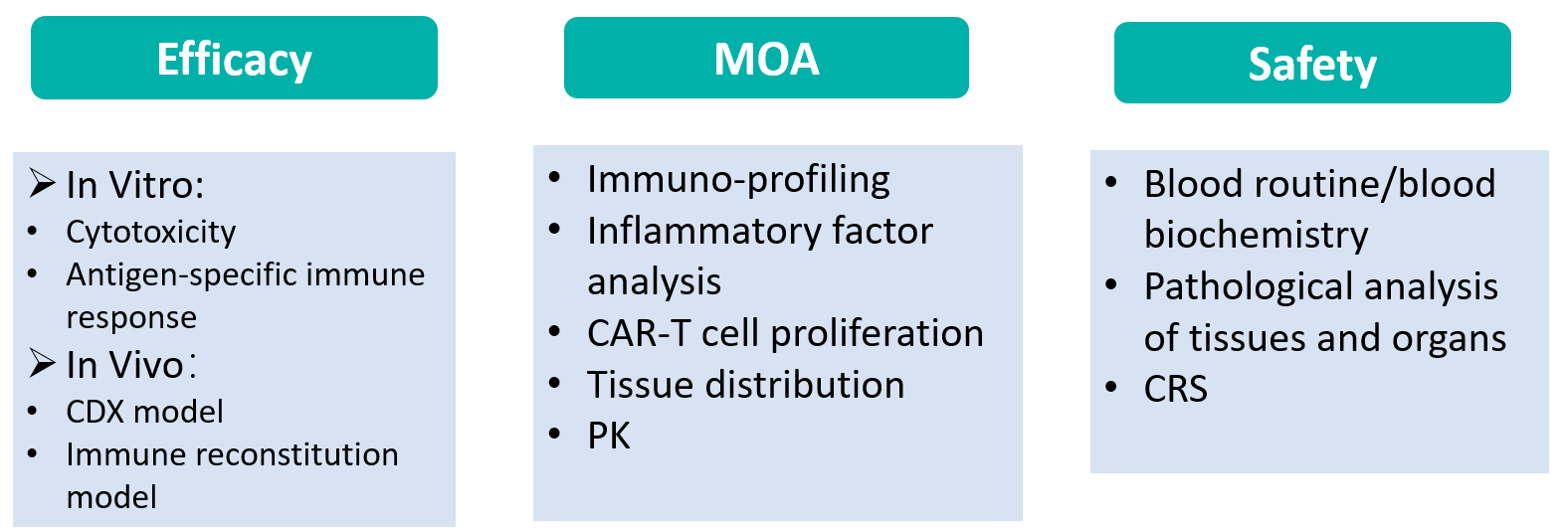
Model selection and tumor cell labeling
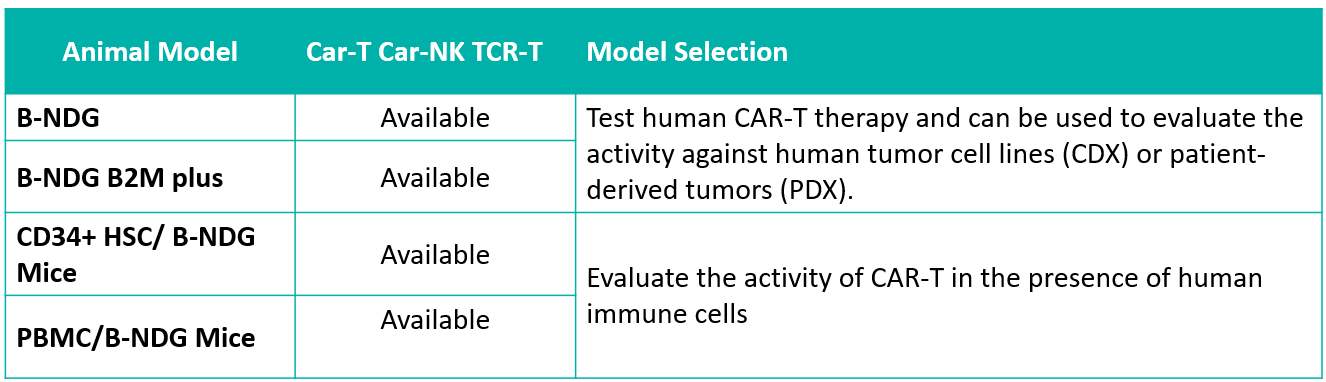
Techniques Involved
-
Bioluminescence imaging of CAR-T cells
-
Flow cytometry analysis of CAR-T cells
-
Histologic analysis
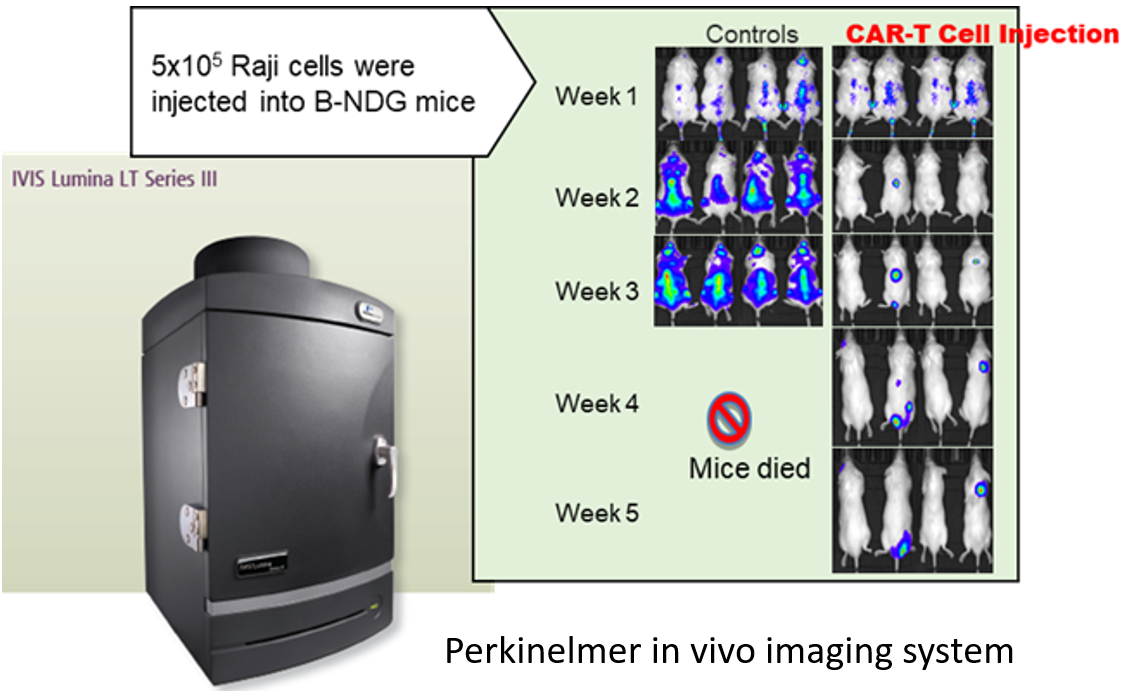
Case: CAR-T in vivo efficacy study using Raji lymphoma CDX tumor model in B-NDG mice
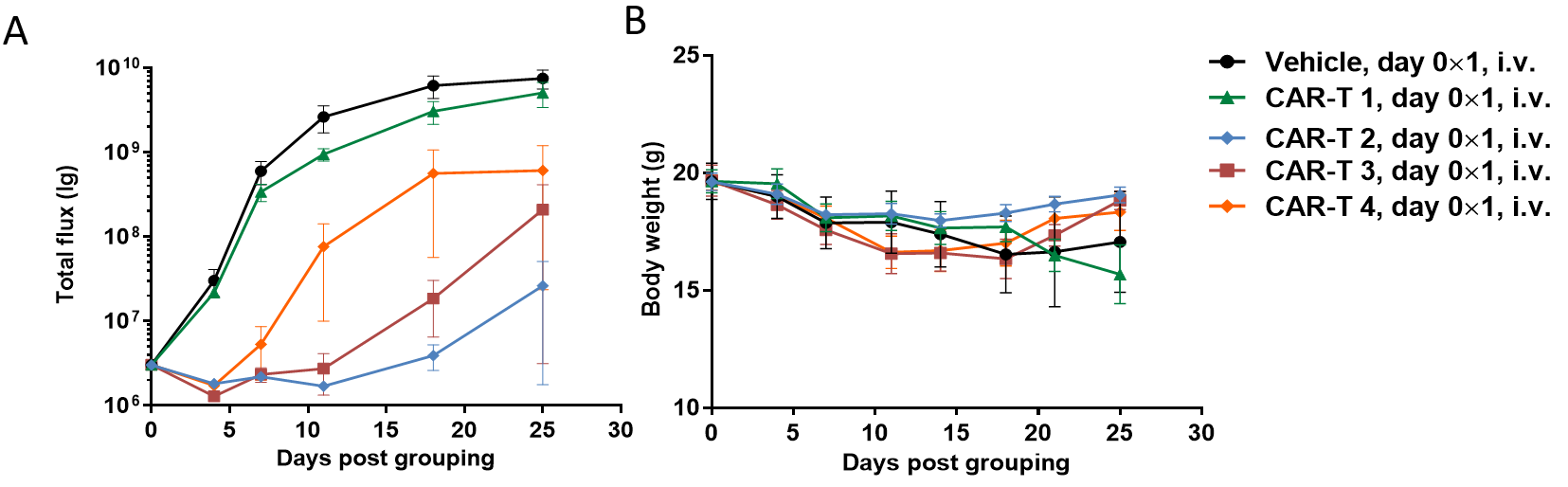
(A) Signal intensity of Raji-Luciferase cell line treated with different CAR-T cells; (B) Body weight changes during treatment.
Case: CAR-T in vivo efficacy study in B-NDG B2m KO mice
Severe xenogeneic graft versus host disease (xeno-GVHD) would shorten treatment window and hamper efficacy evaluation of transplanted cell therapy. β2 microglobulin (B2M) is a small protein as a component of MHC class I molecules, which are present on all nucleated cells, and that are involved in self-recognition and in defense against micro-organisms. So knockout MHC class I β2-microglobulin (β2m) is an effective way to decrease GVHD.
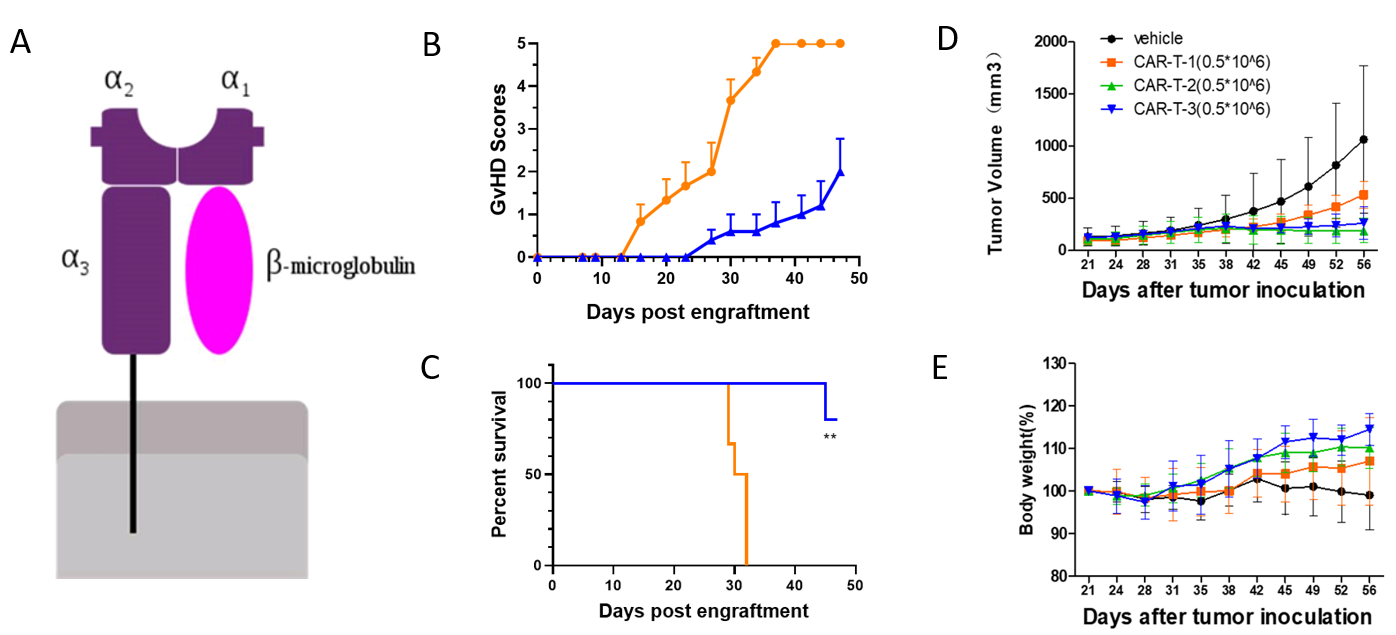
(A) B2M is involved in the composition of MHCI molecules; (B-C) B2M KO mice (blue line) showed decreased GVHD scores and prolonged survival compared to WT B-NDG mice(orange line) after implanted PBMCs; (D-E) CAR-T in vivo efficacy study in B-NDG B2m KO mice, tumor volume(D) and body weight(E).
Case: Car-T proliferation in vivo detected by FACS
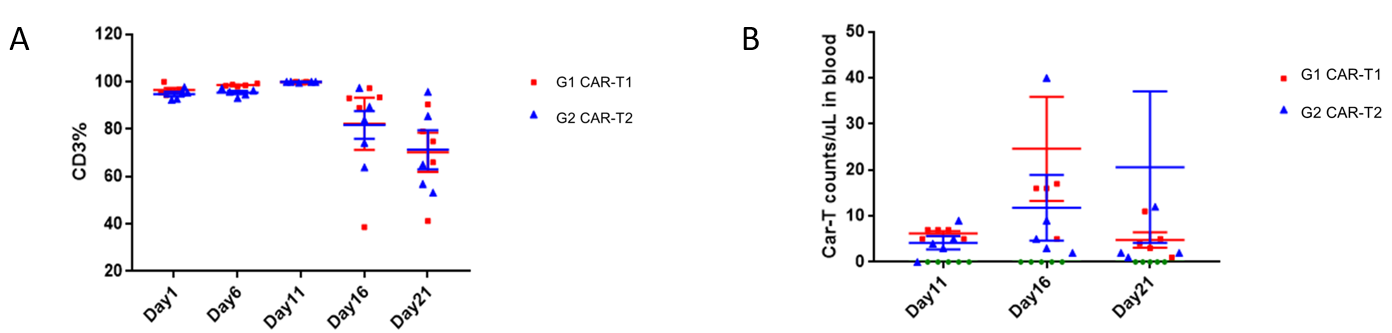
Car-T proliferation in B-NDG mice detected by FACS, the variations of hCD3% (A) and Car-T cell (B) after implantation.
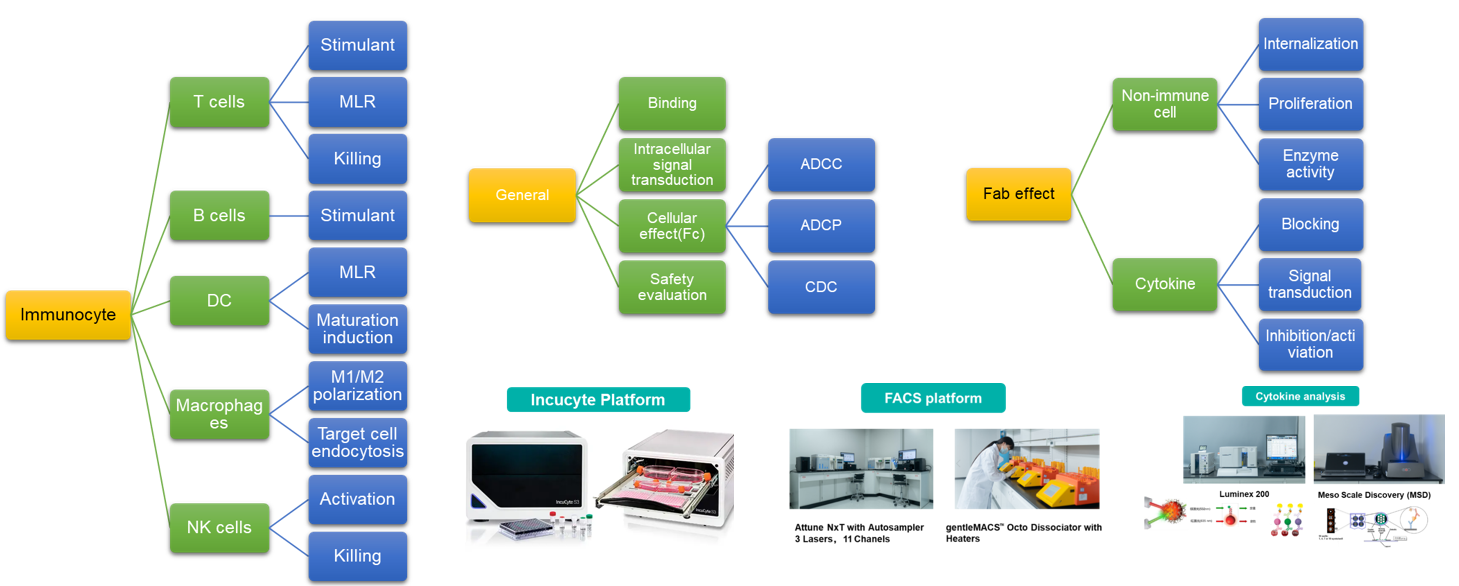
In vitro Pharmacology Platform for cell therapy













 +86-10-56967680
+86-10-56967680 info@bbctg.com.cn
info@bbctg.com.cn